Safeguarding Health Technologies
Bridging science, policy and practice to protect patients

Working together for smarter and safer cardiovascular care
Encouraging innovation while protecting patients
At the European Society of Cardiology, we are deeply committed to ensuring that health technologies that impact cardiovascular care are safe, effective and accessible to all.
Our goal is to foster a regulatory environment that encourages innovation while protecting patients and maintaining the highest standards of safety and efficacy. We bridge European Union (EU) policy and regulation with the real-world expertise of our scientific community by working closely with regulators on medical devices, pharmaceuticals and digital health.
Everything we do to safeguard health technologies is guided by collaboration and compassion. By supporting responsible innovation and championing trust in new technologies, we strive to ensure that every person affected by cardiovascular disease can benefit from cutting-edge technologies.
Championing access, safety and trust with Medical Device Regulation
Dedicated to promoting EU regulations that safeguard patients
Regulation of medical devices is crucial in cardiology, where advanced technologies are essential for patient care. Ensuring these devices are safe, effective, and accessible saves lives.
We actively support EU regulations that protect patients and enable timely access to high-quality cardiovascular devices. Our advocacy has shaped the Medical Device Regulation, emphasising stronger safety standards and a coordinated European approach centered on patients and clinicians.
Since the regulation’s implementation in 2021, we have highlighted challenges that may restrict access to safe innovations. As an observer in the Medical Device Coordination Group (MDCG) and through leadership in the CORE–MD Horizon 2020 project, we contribute to guidance and help regulators balance innovation with safety.
In March 2025, we published a Policy Statement on the regulation's revisions, calling for greater centralisation, stronger clinical involvement, clearer digital health guidance, and enhanced support for high-need cardiovascular devices. We remain committed to ensuring all patients benefit from safe, effective, and innovative medical technologies.

Shaping regulation through shared expertise and strategic thinking
Improving cardiovascular health across Europe
Our approach to medical device regulation is shaped by the insights and experiences of the broader cardiovascular community.
Through strategic think tanks, such as our Cardiovascular Round Table, we bring together representatives from the life sciences sector, patient groups, regulators, clinicians and ESC Board Members to collaboratively identify and discuss ways to improve cardiovascular health across Europe.
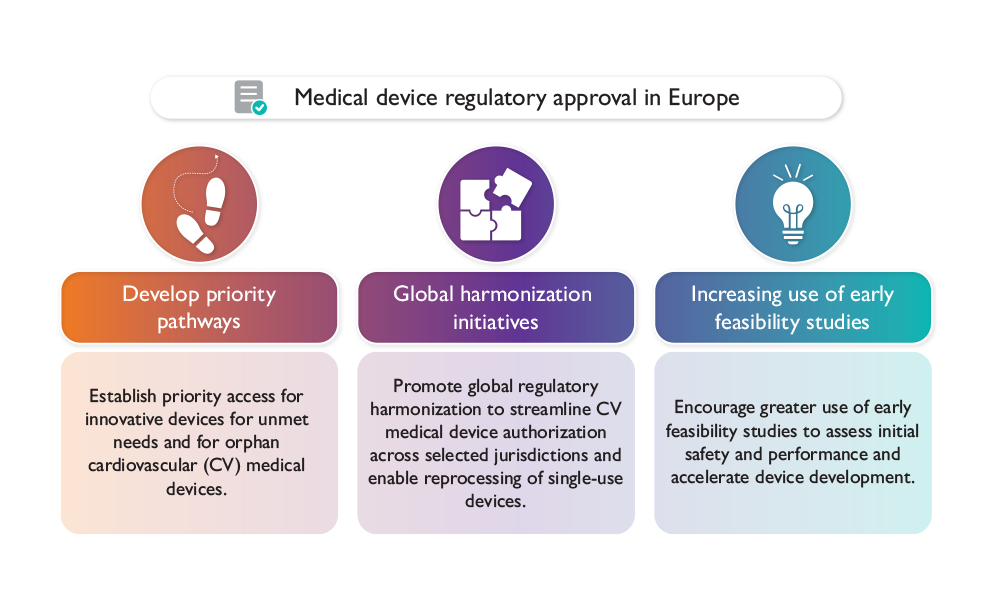
In April 2025, we published a paper summarising the key topics discussed at the second Cardiovascular Round Table meeting on device innovation. This paper highlights strategies to help patients gain timely access to innovative cardiovascular devices that address unmet medical needs, support global harmonisation of regulatory systems and enable faster availability of orphan devices for rare diseases.
Access to medicines and innovation
Advocates for patient-centred regulatory decisions
Our patients rely on timely access to safe, effective and innovative medicines and technologies.
Since 2013, we have been actively sharing clinical expertise, highlighting unmet medical needs and advocating for patient-centred regulatory decisions at the European level through the European Medicines Agency’s Healthcare Professionals’ Working Party (HCPWP).
Through this vital platform, we ensure that the perspectives and needs of cardiovascular patients are heard and represented in EU pharmaceutical policy, regulatory reforms and discussions on medicine shortages.
We also contribute to EU-level discussions on Health Technology Assessment (HTA), providing recommendations from the cardiovascular community for the implementation of the EU HTA Regulation, primarily through our role in the European Commission’s HTA Stakeholder Network.
Our dedication to safe and effective innovation is further demonstrated through participation in EU-funded projects.
















