Smoking increases mortality from all causes and has a crucial role in atherosclerotic cardiovascular disease (ASCVD). It increases the risk of diabetes mellitus and hypertension, and induces lipid oxidation and premature death. Active smoking and second-hand smoke exposure determine more than 30% of coronary heart disease (CHD) mortality [1]. The exact mechanisms of cardiovascular damage are not well known, but the detrimental effect of smoking on endothelial function has long been recognised.
Smoking elicits oxidative processes, and negatively affects platelet function, fibrinolysis, inflammation, and vasomotor function; all these proatherogenic effects double the 10-year risk of fatal events in smokers compared to non-smokers.
Atherosclerotic plaque formation, however, is not thought to be fully reversible and, in terms of ASCVD, smokers would never be expected to reach the risk level of those who have never smoked, especially if they continue to smoke after the age of 30 [2].
Smoking cessation plays an important role in reducing cardiovascular risk, but for heavy smokers there is still a remarkable residual risk after 5 years that should not be underestimated.
Benefits of smoking cessation
Quitting smoking has remarkable and rapid health benefits [3]. Within 20 minutes, the heart and blood pressures drop. At 12 hours, the carbon monoxide level in the blood drops to normal. Within 2 to 12 weeks, the circulation improves, and the lung function increases. Within 1 to 9 months, coughing and shortness of breath decrease. Within 1 year, the risk of CHD is about half that of a smoker. Within 5 years, the risk of stroke is reduced to that of a non-smoker. Within 10 years, the risk of lung cancer falls to about half that of a smoker, and the risk of cancer of the mouth, throat, oesophagus, bladder, cervix or pancreas decreases. Within 15 years, the risk of CHD is that of a non-smoker.
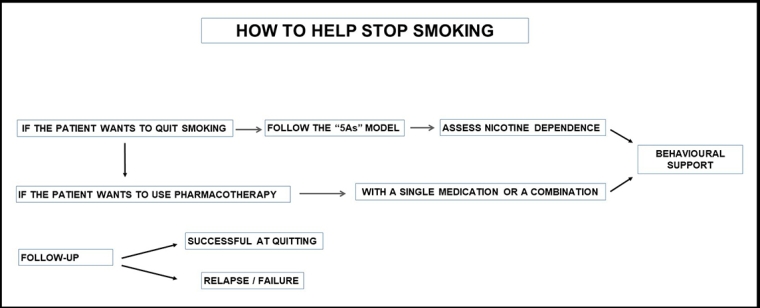
The very first step is identifying who the smokers are in a community or hospital setting and how dependent on nicotine they are, using the Fagerstrom Test for Nicotine Dependence [4] (1-2 = low dependence, 3-4 = low to moderate dependence, 5-7 = moderate dependence, 8+ = high dependence).
Interventions for smoking cessation
Having identified a candidate willing to attempt smoking cessation, in addition to the pharmacotherapeutic and other actions by health professionals (doctors, nurses, counsellors), it is also important to look for help from the person’s family (with their consent) because support from the partner and family can be instrumental.
Brief advice significantly increases cessation rates and is highly cost-effective. However, the most effective approach is a combination of both behavioural support and smoking cessation pharmacotherapy.
One of the barriers mostly frequently cited by health professionals to offering smoking cessation advice is the time required. When time is short, an option is the three-step “Ask, Advise, Help” structure developed by Quit Victoria [5]. This brief intervention model can be summarised as follows:
- Ask and record smoking status
- Advise all people who smoke to quit and inform them of the most effective methods
- Help by offering to arrange referral and encouraging use of behavioural intervention and use of evidence-based smoking cessation pharmacotherapy.
Comprehensive intervention: the 5As approach [2]
If the medical practice has the capacity to offer comprehensive support for cessation, then the 5As approach (Ask, Assess, Advise, Assist, Arrange) provides a structure. It involves:
- Ask – systematically inquire about and document the smoking status of all patients at every opportunity.
- Assess – evaluate nicotine dependence and assess and address barriers to quitting.
- Advise – counsel all patients who smoke to quit in a way that is clear but not confrontational.
- Assist – offer assistance in quitting, agree on a quit plan and recommend pharmacotherapy if the patient is nicotine dependent. If the patient is not willing to quit, use a motivational approach, explore barriers and review at future visits.
- Arrange – for patients making a quit attempt, arrange follow-up contact starting within a week of the quit day. At these visits, congratulate and encourage the patient, review progress and problems, encourage continued use of pharmacotherapy, and monitor and manage any medication side effects.
Barriers to quitting
Most long-term smokers would like to quit, and many have tried repeatedly to do so. There are often various beliefs and attitudes that are barriers to quitting, and it is important to try to identify and, where possible, to offer advice or support to address these. Support could include providing treatment for nicotine withdrawal symptoms or mental health issues or recommending physical activity and a healthy diet to minimise weight gain.
Behavioural interventions
Patients not ready to make a quit attempt may respond to a motivational intervention. The clinician can motivate patients to consider a quit attempt with the "5 Rs" [6]:
- Relevance - encourage the patient to indicate why quitting is personally relevant.
- Risks - ask the patient to identify potential negative consequences of tobacco use.
- Rewards - ask the patient to identify potential benefits of stopping tobacco use.
- Roadblocks - ask the patient to identify barriers or impediments to quitting.
- Repetition - the motivational intervention should be repeated every time an unmotivated patient has an interaction with a clinician. Tobacco users who have failed in previous quit attempts should be told that most people make repeated quit attempts before they are successful.
Tips to stop smoking
Cravings for a cigarette usually last 3 to 5 minutes. If you can get over those few minutes, you are well on the way to not having that cigarette. The 4 Ds can help you to do that.
- Delay - wait at least 3 minutes; the urge will pass.
- Drink - water or juice.
- Distract yourself - move away from the situation and do something different.
- Take deep breaths - breathe slowly and deeply, in through your nose and out through your mouth.
Pharmacotherapy
Different pharmacological strategies are available to treat smoking dependence. They should be associated with non-pharmacological strategies (behavioural counselling), which are validated approaches to promote the process of quitting.
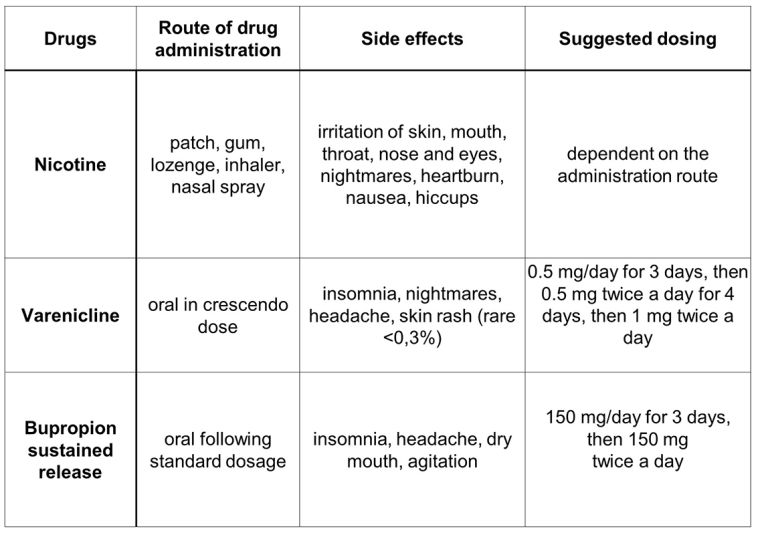
The main pharmacological treatments available for smoking cessation are the following: nicotine replacement therapy (NRT), varenicline, bupropion and cytisine. A network meta-analysis of 63 clinical trials (including 8 trials in CVD patients) found no increase in major adverse cardiovascular events linked to nicotine replacement therapy, bupropion, or varenicline [7].
The clinician should follow both currently available scientific evidence and patient’s preference for a proper choice of one therapy over another, paying particular attention to those patients having contraindications to these drugs due to the presence of specific comorbidities.
Nicotine replacement therapy (NRT)
NRT aims both to stimulate nicotine receptors thus removing smoking craving and withdrawal symptoms (this effect is immediate), and to reduce the number of nicotine receptors (this effect is slower and continues for weeks progressively reducing tobacco dependence) [8]. Each different NRT product has demonstrated the same efficacy in achieving smoking cessation. Consequently, the choice of the NRT product should reflect the patient’s preferences.
A transdermal nicotine patch is commonly the first choice because it yields higher compliance rates than other NRT products [9]. Due to a more beneficial pharmacokinetic profile, combination NRT (short- plus long-acting products) is superior to single NRT. A combination of a transdermal nicotine patch with a more rapidly absorbed NRT (i.e., oro-nasal products) is more effective than the use of a single product [9,10]. Combination NRT is the current standard of therapy once the clinician decides to use NRT as a therapeutic strategy.
NRT usually lasts 12 weeks, although treating heavy smokers for longer intervals may be reasonable, at least until the patient feels confident enough not to relapse. Controlled, longitudinal, and case-controlled trials with NRT in patients with CV diseases did not report greater risks of adverse events compared to placebo [9].
Varenicline
Varenicline is a partial agonist selective for α4β2 nicotinic acetylcholine receptors (nAChRs), one of the receptors related to dopamine release following nicotine binding [11]. Several meta-analyses suggest that varenicline is as effective as combination NRT. These two approaches are considered first-line therapy for smoking cessation, especially in patients with CVD [9]. Smokers should stop smoking one to two weeks after the first dose of varenicline in order to reach the steady state. The pre-medication phase may be prolonged to 4 weeks to increase efficacy [9].
An additional course of 12 weeks’ treatment at 1 mg twice daily may be considered for the maintenance of abstinence in patients who have successfully stopped smoking at the end of the therapeutic interval (12 weeks) [9].
Varenicline could be used safely in patients with stable CVD and with caution in patients with acute coronary syndrome (ACS) [9]. Soon after the introduction of varenicline, reports of an association with depression and suicidal ideation arose. A review of 11 published studies suggested that such association is likely to be weak. Nevertheless, the possibility warrants monitoring patients for clinical and therapeutic evaluation if neuropsychiatric symptoms occur, including behavioural changes, hostility, agitation, depressive mood, and suicidal ideation [12].
Bupropion, sustained release (SR)
When taken to quit smoking, bupropion may confer both anti-craving and anti-withdrawal effects by inhibiting dopamine reuptake, which mediates the reward pathways associated with nicotine use.
Bupropion should be started before the patient’s planned quit day. The patient should set a “target quit date” within the first 2 weeks of therapy and could continue smoking during treatment since this does not significantly affect the pharmacodynamics of bupropion. Steady-state blood levels are usually achieved after 1–2 weeks of treatment. If the patient is not able to quit by the target date, it is possible to delay smoking suspension until the third or fourth week of treatment or when abstinence is reached [8].
Cytisine
Cytisine acts similarly to varenicline. It is a partial agonist selective for α4β2 nicotinic acetylcholine receptors. Cytisine is available as oral tablets containing 1.5 mg of active principle. Clinical data on cytisine found an efficacy similar to, or even higher than NRT regarding the likelihood of smoking cessation. However, cytisine has a greater propensity to adverse events, even though they are mainly minor such as nausea, vomiting, and sleep disorders [9].
Therapeutic efficacy and combination therapy
Combination NRT and varenicline have been associated with higher abstinence rates (33% and 37%, respectively) [13].
Drug therapy for smoking cessation typically lasts 12 weeks. However, these strategies can be personalised based on the clinician and/or patient’s experience. NRT can last longer and even indefinitely, if needed. Similarly, varenicline use can be extended for an additional 3-month period to prevent relapses. Indeed, varenicline was found to be effective and safe if administered for 24 weeks. Long-term use of bupropion is possible, extending the treatment for a further 7–9 weeks [9].
Indeed, the FDA approved both the combination NRT and the combination of single NRT and bupropion for smoking cessation. Combination therapies are justified for patients with a major nicotine addiction or for those who have used monotherapies which have failed because of relapses [9].
Anxiolytics and other antidepressants
Anxiolytics such as benzodiazepines (i.e., low-dose and sustained-release alprazolam) could be helpful for the management of withdrawal symptoms. The most effective and safe antidepressants are selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), atypical antidepressants (i.e., mirtazapine), and serotonin modulators (i.e., trazodone). Due to their proven effectiveness and tolerability, and the amount of available evidence, SSRI should be the first choice [9].
For dosage and adverse events of pharmacotherapy see Figure 1.
Electronic cigarettes
Electronic cigarettes (e-cigarettes) are nicotine delivery devices that use a battery to aerosolize nicotine. Because tobacco is not burned, these devices are likely to be safer than continuing to smoke conventional tobacco cigarettes.
The use of e-cigarettes is considered to be a reduced-harm alternative to conventional cigarettes, but they are not harm-free. Newer devices can deliver higher nicotine contents, and e-cigarettes emit other constituents such as carbonyls and fine and ultrafine particulates [2].
However, their safety with long-term use is uncertain because they are newer products that have not yet been evaluated, nor have they been approved for smoking cessation by the US Food and Drug Administration (FDA) Center for Tobacco Products [10].
Many e-cigarette products are available that vary in the amount of nicotine delivery. The role of e-cigarettes in smoking cessation treatment is unclear; results of randomised controlled trials suggest that they have efficacy, but the number of studies are few and the devices themselves are evolving rapidly. The role in smoking cessation treatment is unclear also for heat-not-burn tobacco cigarettes, which are another type of electronic nicotine delivery system.
Special situations
Psychiatric illness
For patients with comorbid psychiatric disease, we suggest initiating varenicline rather than NRT.
Cardiovascular disease
In patients with stable CVD, we use the same treatments as those for the general population. Varenicline, NRT, and bupropion are effective in this population.
Acute coronary syndrome
In patients hospitalised with ACS, the safety of smoking cessation medications, rather than their efficacy, is the major consideration since there is no a priori reason to assume that their efficacy would differ compared with stable CVD. There are fewer data regarding their efficacy and safety in smokers hospitalised with ACS, but available studies suggest that they are probably safe to use.
Hospitalised smokers
NRT is often used to treat nicotine withdrawal symptoms in hospitalised smokers because it has a rapid onset of action, whereas varenicline and bupropion are slower to reduce nicotine withdrawal symptoms
Pregnant smokers
The use of NRT during pregnancy is controversial because nicotine has been shown to have foetotoxicity in animal models. In addition, previous randomised controlled trials have been inconclusive with respect to the efficacy and safety of NRT on cessation rates among pregnant smokers. A randomised, placebo-controlled trial with a maximum of 2 months of nicotine patch exposure during the entire pregnancy found neither an increased abstinence rate nor higher birth weight with the nicotine patch as compared with a placebo patch. Similarly, there was no difference in safety. Because of the lack of randomised controlled studies among pregnant smokers, neither bupropion nor varenicline is indicated during pregnancy [13].
Follow-up and management of relapse
It is necessary to schedule a follow-up visit (e.g., telemedicine encounter, telephone call, or in-person office visit) in one to two weeks to monitor for adverse effects, reinforce adherence to medication, and provide support for smoking cessation [10].
Further follow-up to assess for new side effects, smoking cessation, or relapse should be scheduled at three months and at one year, and more frequently if necessary.
Assessment for persistent smoking
If a patient does not stop smoking after two to four weeks of pharmacotherapy, one or more of the following may be occurring: incorrect use of medication(s), intolerance of side effects, persistent nicotine withdrawal symptoms despite correct use of the medication. For patients who continue to experience withdrawal symptoms despite correct use of a first-line pharmacotherapy, we suggest adding short-acting NRT. In addition, the dose of the first-line medication should be increased if it is not yet maximised.
- If there has been no response to the initial agent, we switch to a different first-line pharmacotherapy.
- If the smoker has had a partial response to the initial medication (i.e., reducing smoking but not quitting altogether), adding an additional medication is reasonable. In particular, for smokers who have had partial success with varenicline, adding a nicotine patch may increase smoking abstinence.
Duration of pharmacotherapy
In general, pharmacotherapy for smoking cessation is recommended for at least three months [10].
Both varenicline and bupropion use may be extended up to one year if the patient has quit smoking but still feels at risk of relapse based upon experience with prior quit attempts. If bupropion is also being used to treat mood, continuation of therapy should take into consideration management of depressive symptoms. NRT may be extended and even used indefinitely if needed.
For patients who successfully quit smoking and then experience relapse, we suggest restarting the pharmacologic agent(s) that previously worked for the patient. This may be enhanced with more intensive behavioural support and/or intensified pharmacotherapy (e.g., adding another medication) [9].
A flow chart of smoking cessation is provided in Figure 2.
Conclusion
Cigarette smoking is the number one cause of diseases that can be effectively prevented. Cigarette smoking is not only a risk factor for chronic CVD, but it is also an inducer of acute atherothrombotic events such as stroke or myocardial infarction and has an impact on glucose tolerance and levels of HDL cholesterol. There is strong evidence that a range of pharmacologic and behavioural interventions, both individually and in combination, is effective in increasing smoking cessation. Pharmacological therapies help smokers adjust to the absence of nicotine following cessation of smoking by lessening the symptoms of nicotine withdrawal.