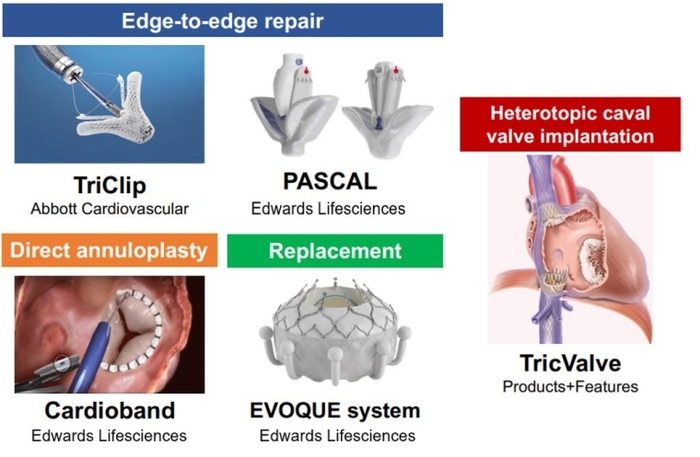
Transcatheter edge-to-edge repair
Tricuspid transcatheter edge-to-edge repair (T-TEER) techniques aim to increase leaflet coaptation by grasping tricuspid valve leaflets. T-TEER devices have been developed based on the initial experience of applying mitral TEER to the tricuspid valve [5,6] (Table 1). The latest generation of TriClip (TriClip G4 system; Abbott) has multiple clip sizes and allows independent leaflet grasping in order to optimise leaflet insertion. This has improved procedural results as shown in the latest data from the bRIGHT post approval study (TR reduction to ≤moderate at 30 days in 77% of the patients) [7].
Similarly, the PASCAL system (Edwards Lifesciences) has shown encouraging results in early feasibility studies in the EU and USA [8,9]. The PASCAL system consists of a central spacer that acts as a filler in the regurgitant orifice of the TV, while its nitinol construction may minimise the stress on fragile tricuspid leaflets.
Owing to its broad availability and high safety, T-TEER is the most widely used transcatheter technique for TR in the current clinical environment. The TRILUMINATE Pivotal trial – the first randomised control trial comparing T-TEER and medical therapy alone – revealed that T-TEER with the TriClip system improved quality of life in patients with TR [10]. On the other hand, clinical events, such as mortality or hospitalisation due to heart failure, were comparable between the T-TEER and medical therapy groups. Several other ongoing randomised control trials, including the CLASP II TR trial (the PASCAL system vs medical therapy), will provide further insights into the benefits of T-TEER during the year 2024 (Table 2).
Table 1. Key trials of CE-marked transcatheter devices for TR.
Edge-to-edge repair:
- TRILUMINATE Feasibility Study (NCT03227757)
- Design: Prospective, single-arm, observational study
- Device: TriClip NT
- Number of patients: 85
- TR reduction at 30 days: At least 1 grade reduction in 86%; TR reduction to ≤2+ in 56%
- Post-procedural mortality: 6-month mortality 5%
- Clinical outcomes: Improvement in NYHA class, KCCQ score, and 6MWD; 40% reduction in hospitalization
- CLASP TR Early Feasibility Study (NCT03745313)
- Design: Prospective, single-arm, observational study
- Device: PASCAL
- Number of patients: 34
- TR reduction at 30 days: At least 1 grade reduction in 85%; TR reduction to ≤2+ in 52%
- Post-procedural mortality: 30-day mortality 0%
- Clinical outcomes: Improvement in NYHA class, KCCQ score, and 6MWT at 30 days
- bRIGHT Post-Approval Study (NCT04483089)
- Design: Prospective, post-market survey
- Device: TriClip G4
- Number of patients: 511
- TR reduction at 30 days: TR reduction to ≤2+ in 77%
- Post-procedural mortality: 30-day mortality 1.0%
- Clinical outcomes: Improvement in NYHA class and KCCQ score
- TRILUMINATE Pivotal Study (NCT03904147)
- Design: Prospective randomized control trial (control arm: medical therapy alone)
- Device: TriClip G3 or G4
- Number of patients: 350 (175 for intervention)
- TR reduction at 30 days: TR reduction to ≤2+ in 87%
- Post-procedural mortality: 30-day mortality 0.6%
- Clinical outcomes: Improvement in KCCQ score compared to medical therapy alone at 1 year
Direct annuloplasty:
- TRI-REPAIR Study (NCT02981953)
- Design: Prospective, single-arm, observational study
- Device: Cardioband
- Number of patients: 30
- TR reduction at 30 days: TR reduction to ≤2+ in 76%
- Post-procedural mortality: 30-day mortality 6.7%
- Clinical outcomes: Improvement in NYHA class, KCCQ score, and 6MWT at 6 months
- Cardioband EFS (NCT03382457)
- Design: Prospective, single-arm, observational study
- Device: Cardioband
- Number of patients: 37
- TR reduction at 30 days: TR reduction to ≤2+ in 45.4%
- Post-procedural mortality: 30-day mortality 0%
- Clinical outcomes: Improvement in NYHA class and KCCQ score at 1 year
- TriBAND Study (NCT03779490)
- Design: Prospective, post-market survey
- Device: Cardioband
- Number of patients: 61
- TR reduction at 30 days: TR reduction to ≤2+ in 69%
- Post-procedural mortality: 30-day mortality 1.6%
- Clinical outcomes: Improvement in NYHA class and KCCQ score at 30 days
Caval valve implantation:
- TRICUS EURO study (NCT04141137)
- Design: Prospective, single-arm, observational study
- Device: TricValve
- Number of patients: 35
- TR reduction at 30 days: Not reported
- Post-procedural mortality: 30-day mortality 5.7%
- Clinical outcomes: Improvement in NYHA class and KCCQ at 6 months
Replacement:
- TRISCEND Study (NCT04482062)
- Design: Prospective, single-arm, observational study
- Device: EVOQUE system
- Number of patients: 56
- TR reduction at 30 days: TR reduction to ≤1+ in 98.1%
- Post-procedural mortality: 30-day mortality 3.6%
- Clinical outcomes: Improvement in NYHA class, KCCQ score, and 6MWT at 30 days
- TRISCEND II Pivotal trial (NCT04482062)
- Design: Prospective randomized control trial (control arm: medical therapy alone)
- Device: EVOQUE system
- Number of patients: First 150 (96 for intervention)
- TR reduction at 6 months: TR reduction to ≤1+ in 93.8%
- Post-procedural mortality: 30-day cardiovascular mortality 3.2%
- Clinical outcomes: Improvement in NYHA class, KCCQ score, and 6MWT at 6 months
KCCQ: Kansas City Cardiomyopathy Questionnaire; NYHA: New York Heart Association; TR: tricuspid regurgitation; 6 MWT: 6-minute walk test
Table 2. Upcoming landmark studies of transcatheter tricuspid valve interventions.
Randomised control trials:
- CLASP II TR
- Type of intervention: T-TEER
- Country: US
- Registration: NCT04097145
- Study arm: PASCAL + OMT vs. OMT (1:1)
- Enrollment: 870
- Primary endpoint: Composite of mortality, RVAD implantation or heart transplant, TV intervention, HF hospitalization, and QoL improvement at 24 months
- TRI-FR
- Type of intervention: T-TEER
- Country: France
- Registration: NCT04646811
- Study arm: T-TEER + OMT vs. OMT (1:1)
- Enrollment: 300
- Primary endpoint: Composite of NYHA class, patient global assessment, and major cardiovascular events at 12 months
- TRICI-HF
- Type of intervention: Any devices
- Country: Germany
- Registration: NCT04634266
- Study arm: Any CE-marked devices + OMT vs. OMT (2:1)
- Enrollment: 360
- Primary endpoint: Composite of mortality and HF hospitalization at 12 months
- TRISCEND II Pivotal trial
- Type of intervention: TTVR
- Countries: US and EU
- Registration: NCT04482062
- Study arm: EVOQUE + OMT vs. OMT (2:1)
- Enrollment: 1,070
- Primary endpoint: Composite of mortality, RVAD implantation or heart transplant, TV intervention, HF hospitalization, NYHA class, and 6MWD
Feasibility studies:
- TARGET
- Type of intervention: TTVR
- Country: Germany
- Registration: NCT05486832
- Study arm: Single-arm: Cardiovalve
- Enrollment: 100
- Primary endpoint: Freedom from device or procedure-related adverse events within 30 days
- TTVR Early Feasibility Study
- Type of intervention: TTVR
- Country: US
- Registration: NCT04433065
- Study arm: Single-arm: Intrepid
- Enrollment: 15
- Primary endpoint: Rate of implant or delivery-related serious adverse events
- Study to Evaluate the Safety and Performance of LuX-Valve Plus System for TR
- Type of intervention: TTVR
- Country: France
- Registration: NCT05436028
- Study arm: Single-arm: Lux-Valve Plus
- Enrollment: 135
- Primary endpoint: Composite of Major Adverse Event (MAE) at 30 days postprocedure
- Trisol EFS Study
- Type of intervention: TTVR
- Country: US
- Registration: NCT04905017
- Study arm: Single-arm: Trisol
- Enrollment: 15
- Primary endpoint: Rate of device-related serious adverse events
- TANDEM I
- Type of intervention: Spacer
- Country: Poland
- Registration: NCT05296148
- Study arm: Single-arm: CroiValve
- Enrollment: 10
- Primary endpoint: Freedom from device or procedure-related serious adverse events
- Feasibility Study of the DragonFly-T System for Severe TR
- Type of intervention: T-TEER
- Country: China
- Registration: NCT05671640
- Study arm: Single-arm: DragonFly-T
- Enrollment: 10
- Primary endpoint: Composite of all-cause death or recurrent HF hospitalizations within 12 months
- TriStar
- Type of intervention: Indirect annuloplasty
- Country: China
- Registration: NCT05173233
- Study arm: Single-arm: K-Clip
- Enrollment: 67
- Primary endpoint: All-cause mortality and change of TR grade at 1 year
- TRIBUTE
- Type of intervention: Leaflet approximation
- Country: Israel
- Registration: NCT05767645
- Study arm: Single-arm: Mistral device
- Enrollment: 75
- Primary endpoint: Incidence of major device-related adverse events at 6 months
CE: European conformity; HF: heart failure; KCCQ: Kansas City Cardiomyopathy Questionnaire; NYHA: New York Heart Association; OMT: optimal medical therapy; QoL: quality of life; RVAD: right ventricle assist device; TR: tricuspid regurgitation; TV: tricuspid valve; 6 MWT: 6-minute walk test
Direct annuloplasty
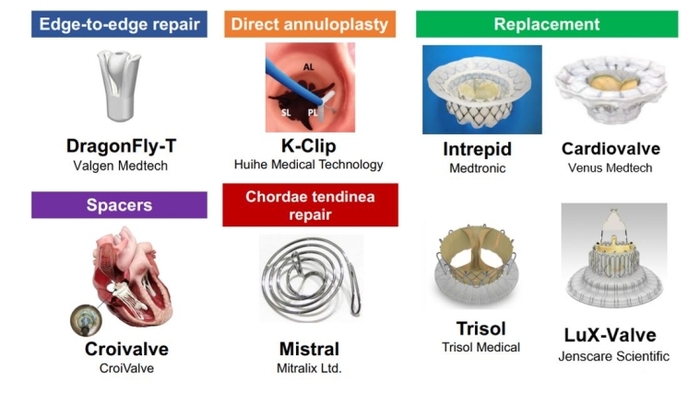
Tricuspid annulus dilation reduces leaflet coaptation and represents the main mechanism of TR progression. Direct annuloplasty devices mimic surgical tricuspid annuloplasty and aim to reduce tricuspid annular size. The Cardioband system (Edwards Lifesciences) is the first device to receive a CE (Euorpean conformity) mark as a direct annuloplasty device for the tricuspid valve position. The implantation is performed by means of multiple screw anchors and can be cinched to improve leaflet coaptation through downsizing of the tricuspid annulus. Patients with TR primarily due to tricuspid annulus dilation may be good candidates for direct annuloplasty devices. Furthermore, the direct annuloplasty allows a combined procedure with T-TEER, which may be a promising treatment option for patients with progressive TR. Early feasibility studies in the EU and USA revealed the safety and effectiveness of TR reduction by the Cardioband system (Table 1) [11,12]. A European post-market follow-up study (the TriBAND study) showed the effectiveness of the Cardioband system in real-world practice [13]. In addition, a novel direct annuloplasty technology, K-Clip (Huihe Medical Technology), which reduces the tricuspid annulus circumference by directly grasping the tricuspid annulus by a clip, is prepared for clinical trials [14].
Transcatheter tricuspid valve replacement
Despite the multiple options of repair devices, there remain concerns about significant residual TR in a non-negligible number of patients. Transcatheter tricuspid valve replacement (TTVR) may be a key treatment option to tackle this unmet need. Currently, the EVOQUE tricuspid valve replacement system (Edwards Lifesciences) is the first CE-certified TTVR system consisting of a self-expanding bioprosthetic valve that can be delivered percutaneously via the transfemoral transvenous approach. The prospective TRISCEND study reported feasibility, safety, and effectiveness of the EVOQUE system (Table 1) [15]. The ensuing TRISCEND II Pivotal Trial, a randomised control trial comparing clinical outcomes between the EVOQUE system and conservative treatment of 150 patients, demonstrated that TTVR with the EVOQUE system improved quality of life and functional capacity at six months, compared to medical therapy alone (Table 2). Notably, the EVOQUE system achieved TR reduction to ≤mild in 93.8% of the patients. This ability to eliminate TR is one of the major advantages, which may lead to better clinical outcomes. In addition, several other TTVR systems such as Cardiovalve (Venus Medtech), Intrepid (Medtronic), LuX-Valve (Jenscare Scientific) and Trisol (Trisol Medical) are currently under clinical investigation (Table 2), and promising results from first-in-human series have shown the feasibility, safety, and efficacy of these TTVR devices.
Caval valve implantation
Heterotopic tricuspid valve replacements, meaning stented valve implantation in the inferior and superior vena cava (bicaval valve implantation), are alternative treatment options in cases of far advanced TR with subsequent right ventricular remodelling and annular dilatation. Caval valve implantation can reduce backflow into the vena cava and may improve symptoms related to the venous congestion. The TricValve system (Products + Features) is a CE (European conformity)-marked device for caval valve implantation, consisting of two self-expanding valves designed for the superior and inferior vena cava. An early feasibility study (the TRICUS EURO) (Table 1) showed improvement in heart failure symptoms and quality of life after TricValve implantation [16]. However, heterotopic tricuspid valve replacement remains a bailout option for patients with anatomy ineligible for other transcatheter devices or inadequate echocardiographic imaging quality.
Postinterventional management after transcatheter tricuspid valve intervention
Transcatheter tricuspid valve repair techniques, such as T-TEER and direct annuloplasty, require life-long antiplatelet therapy after the procedures, usually with aspirin – as long as there is no other indication for oral anticoagulation. The vast majority of patients who undergo tricuspid valve interventions suffer from concomitant atrial fibrillation, and, therefore, oral anticoagulation can be continued without the need for adaptation of the treatment regimen. For patients undergoing TTVR, life-long oral anticoagulation is recommended to prevent prosthetic valve thrombosis. However, the most appropriate regimen is not yet established. The early studies of TTVR used vitamin K antagonists as the postprocedural anticoagulant treatment, while direct oral anticoagulants are recommended in the TRISCEND II trial. Since patients with TR are generally considered to be at high bleeding risk due to multiple comorbidities, further investigations are certainly needed.
During postprocedural follow-up, special attention should be paid to potential changes in volume status. Owing to a decreased venous backflow and an elevated right ventricular (RV) forward stroke volume, the venous congestion may rapidly improve, wherein reduction of diuretics might be necessary. Moreover, regular echocardiographic follow-ups should be performed to monitor right heart function and pulmonary pressures as well as dynamics of left-sided valvular diseases, which in turn might be influenced by an elevated RV forward stroke volume.
Impact on practice statement
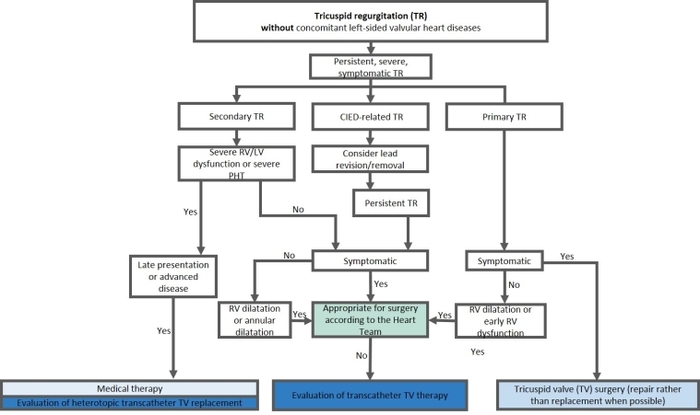
Tricuspid regurgitation is a common valvular disease leading to a significant reduction of functional capacity and is associated with excess morbidity and mortality – if left untreated. Multiple surgical and transcatheter treatment options are currently available for TR treatment. Data regarding safety and symptomatic improvements of transcatheter treatment are encouraging. Currently ongoing randomised control trials will show the impact of these procedures on life expectancy.