Keywords
chemotherapy, radiotherapy, toxicity, follow-up, cardio-oncology
Abbreviation list
CPET - cardiopulmonary exercise test
CS - cancer survivors
cTn - cardiac troponins
CTR-CVT - cancer treatment-related cardiovascular toxicity
CVD - cardiovascular disease
ESC -European Society of Cardiology
GLS - global longitudinal strain
HFA - Heart Failure Association of the ESC
LVEF - left ventricular ejection fraction
ICOS - International Cardio-Oncology Society
MDT - multidisciplinary team
NP - natriuretic peptide
NMR - nuclear magnetic resonance
PAD - peripheral artery disease
RT - radiotherapy
Take-home messages
- Every CS at end of therapy should receive a careful evaluation of the risk of future CTR-CVT development.
- The risk factors for the development of future CRT-CVT at the end of therapy are related to the patient’s basal risk level, to the toxicity of the cancer therapy, to the development of a CTR-CVT during treatment and to the results of the clinical and instrumental findings at the end of therapy and should periodically evaluated with a clinical assessment, ECG, echocardiography, and biomarkers.
- Specific recommendations apply for the management of patients at the end of therapy according to their risk level.
Patient-oriented messages
- Some cancer treatments may negatively, albeit infrequently, affect the heart and circulation. These rare side effects can be prevented if correctly diagnosed and treated.
- You should understand the importance of a careful CV assessment at the end of treatment and eventually, at one year and long-term follow-up.
- This evaluation consists of simple cardiac consultations and sometimes of echocardiography and blood exams to evaluate your risk of developing a CTR-CVT.
- If you are considered at low risk and your cancer treatment was potentially cardiotoxic, you may need one heart check in the first year after the end of therapy.
- If you are considered at moderate risk and your cancer treatment was potentially cardiotoxic you need closer monitoring of your heart health with more frequent tests after treatment.
- If you are considered at high risk and your cancer treatment was potentially cardiotoxic you will need a more articulated one year and long-term follow-up. The ESC Guidelines provide information to your physicians on which protective treatments to consider and which tests to carry out during the first year after treatment and the long-term.
Impact on practice statement
Every physician taking care of a patient at the end of cancer treatment should know how to identify patients who will need further cardiology assessments to identify future CVD. On the other hand, it helps to avoid useless screening of cancer populations without any CV risk.
The risk assessment includes:
- the HFA-ICOS assessment tools [2] (Table 1)
- the kind of cancer therapy and its specific risk of long-term CRT-CVT
- the development during treatment of a moderate or severe CRT-CVT
- the development of new symptoms or cardiac abnormalities evaluated by echocardiography or serum biomarkers (at 3 months in high-risk patients) and at 1 year in any risk categories.
This evaluation also allows decision on continuing or weaning off CV protective treatments.
CTR-CVT: cancer treatment-related cardiovascular toxicity; CV: cardiovascular; CVD: cardiovascular disease; CS: cancer survivor; LV: left ventricular; MDT: multidisciplinary team
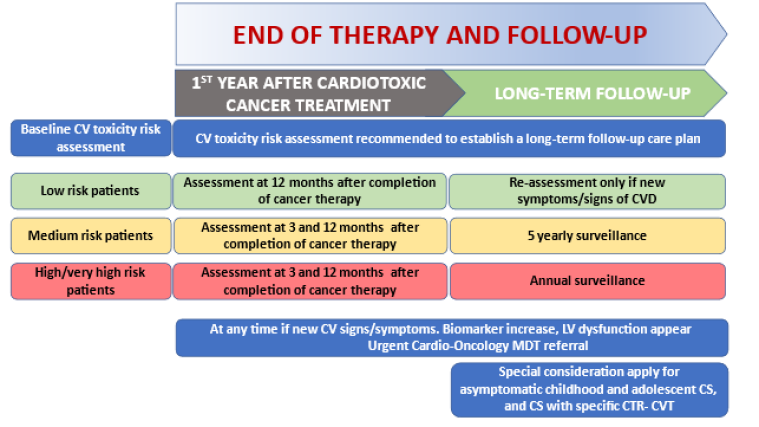
1. CTR-CVT risk evaluation at the end of therapy
1.1 Defining the “end of therapy”
End of cancer therapy begins immediately after the last cardiotoxic cancer therapy dose administration. It is not possible to define the ‘end of therapy’ in cases of discontinuation of therapy for any reason, cancer progression, poor prognosis or end of life care.
Moreover, in some conditions, patients may receive complex neoadjuvant therapies associated with radiotherapy (RT) at the beginning of cancer therapy and continue the adjuvant therapies for very long periods (e.g., trastuzumab and long-term antioestrogen therapy in breast cancer: the end of therapy coincides here with the last trastuzumab dose).
1.2 Tools for CTR-CVT risk evaluation at the end of therapy
1.2.1 Clinical evaluation
The first step in the evaluation at the end of therapy consists of a careful clinical history and physical examination that should be undertaken by oncology specialists or any kind of medical professional who has a CS in their charge. The CS population may be divided in two groups: normal patients and patients with a pre-existing CVD or with a pre-existing CTR-CVT leading to a prevention strategy with specific interventions during therapy and also at the end of therapy.
This evaluation includes a re-evaluation every 12 months (and at 3 months for high-risk patients) for common cardiovascular (CV) risk factors and preventive measures.
Although not specific to cancer patients, the SCORE2 and SCORE2-OP [3] are suggested for risk evaluation and consequent optimisation in CS >40 years of age. Relevant risk factors are a family history of premature cardiovascular disease (CVD), lifestyle factors (smoking, alcohol consumption, sedentary habits, exposure to pollution), and frailty; and moreover, a prior treatment history and history of doses of cardiotoxic drugs.
Typical cardiac symptoms and signs like dyspnoea, chest pain, valvular murmurs and signs of congestion suggesting the presence of an unknown CVD, require specific investigations. .
A prior history of CVD or of CTR-CVT requires a more comprehensive evaluation for the possible development and evolution of new future CVD or CTR-CVT.
1.2.2 Imaging
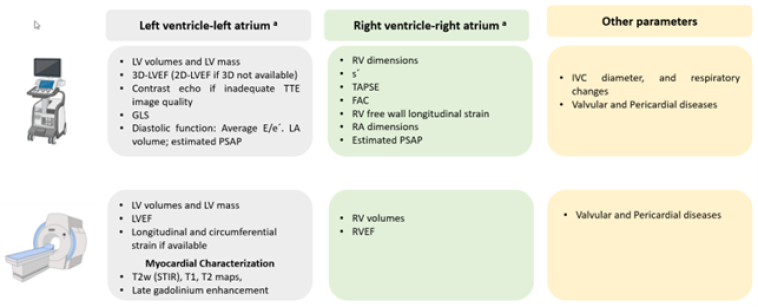
An echocardiographic assessment of left and right ventricular function may identify the level of risk of patients at the end of therapy. Echocardiography may be done by the traditional 2D method, and more recently, by 3D echo and speckle tracking, allowing for the evaluation of the global longitudinal strain (GLS) of both ventricles [3]. In case of technical problems such as poor image quality, other imaging techniques may be used. Nuclear magnetic resonance (NMR) is the most promising although relatively unavailable and costly.
Figure 2 shows the parameters which may be obtained by echo and NMR.
FAC: fractional area change: GLS: global longitudinal strain; IVC: inferior vena cava : LA: left atrium; LV: left ventricular; LVEF: left ventricular ejection fraction; PSAP: pulmonary systolic artery pressure: RA: right atrium; RV: right ventricular; RVEF: right ventricular ejection fraction; s’: TAPSE: TTE: transthoracic echocardiography;
The measurement at the end of therapy and at one year of natriuretic peptides (NPs) and cardiac troponins (cTn) [4] has a high negative predictive value for future CTR-CVT end events. In a large study, the incidence of cardiotoxicity after anthracycline was 9% and about 98% of cases were diagnosed at a median time of 3.5 months [5]. Each 1% decrease of the left ventricular ejection fraction (LVEF) predicted CTR-CVT with a hazard ratio (HR) 1.37, 95% confidence interval (CI): 1.33–1.42.
The risk of future events may also be detected by the determination of cTn at the end of cancer therapy, e.g., after anthracycline chemotherapy an increase in cTnI level identifies CS at risk for developing LV dysfunction and which patients should receive angiotensin-converting enzyme inhibitors (ACE-I) and/or betablocker protection.
1.3 Risk factors for future cardiovascular diseases at the end of cancer therapy
- Baseline risk (assessed by Heart Failure Association of the ESC (HFA)- International Cardio-Oncology Society (ICOS) risk assessment tools) [2]
This risk assessment is based on CV risk factors, on previous CVD history, on the cancer story and on previous cancer treatments. This assessment implies a clinical assessment, an electrocardiogram (ECG), and in the case of pre-existing CVD or cancer treatments entailing a high-risk of CTR-CVT, the determination of cTn, NPs and of LVEF and GLS with echocardiogram.
- Type (and dosage) of cancer therapy
Each chemotherapy agent may entail a specific risk of CTR-CVT, from no risk to very high risk, generally related to the doses administered, both single doses and cumulative doses (e.g. cumulative doses of anthracyclines), and to the contemporary or in-sequence administration of other cancer treatments (e.g. anthracyclines plus trastuzumab plus RT).
- CRT-CVT occurring during therapy administration
Includes almost every kind of CV damage at any CV level, of different pathophysiological meaning and of different clinical relevance.
- Clinical conditions, echocardiography, and biomarkers assessment
This reappraisal should be undertaken in all CS at 12 months and at 3 and 12 months in patients classified at high and very high risk at baseline. The echocardiography should include a complete evaluation of left and right ventricle function and, when possible, the evaluation of the GLS compared with baseline data. The clinical evaluation should include CV risk factors according to the ESC Guidelines on Prevention [6].
Table 1 shows the baseline risk stratification according to previous CVD, imaging findings, biomarkers, age and CV risk factors, lifestyle risk factors, current or previous exposure to anthracyclines and RT, in relationship with five of the most common treatments.
Table 1. Baseline CV toxicity risk stratification.
With permission from [1]. Lyon AR, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43:4229-361.
Recovery from myocarditis
Baseline CV Toxicity Risk Factors by Treatment
-
Previous cardiovascular disease (CVD)
-
Heart failure (HF)/cardiomyopathy/CTRCD
- Very high risk (VH): Anthracycline, HER2 therapies, VEGF inhibitors, Multiple myeloma therapies, RAF-MEK inhibitors
- High risk (H): BCR-ABL inhibitors
-
Severe valvular heart disease
- High risk: Anthracycline, HER2 therapies, RAF-MEK inhibitors
-
MI or PCI or CABG
- High risk: Anthracycline, HER2 therapies, RAF-MEK inhibitors
- Very high risk: VEGF inhibitors
-
Stable angina
- High risk: Anthracycline, HER2 therapies, RAF-MEK inhibitors
- Very high risk: VEGF inhibitors
-
Arterial vascular disease
- Very high risk: VEGF inhibitors, BCR-ABL inhibitors, Multiple myeloma therapies
-
Abnormal ABPI
- High risk: BCR-ABL inhibitors
-
Pulmonary hypertension
- High risk: BCR-ABL inhibitors
-
Arterial thrombosis with TKI
- Very high risk: BCR-ABL inhibitors
-
Venous thrombosis (DVT/PE)
- High risk: VEGF inhibitors
- Moderate risk (M2): BCR-ABL inhibitors
- Very high risk: Multiple myeloma therapies
-
Arrhythmias
- Moderate risk (M2): HER2 therapies, VEGF inhibitors, BCR-ABL inhibitors, Multiple myeloma therapies
- Moderate risk (M1): RAF-MEK inhibitors
-
QTc ≥480 ms
- High risk: VEGF inhibitors, BCR-ABL inhibitors
-
450 ms ≤ QTc <480 ms (men), 460 ms ≤ QTc <480 ms (women)
- Moderate risk (M2): VEGF inhibitors, BCR-ABL inhibitors
-
Prior PI CV toxicity
- Very high risk: Multiple myeloma therapies
-
Prior IMID CV toxicity
- High risk: Multiple myeloma therapies
-
LVEF <50%
- High risk: All treatment types
-
LVEF 50–54%
- Moderate risk (M2): Anthracycline, HER2 therapies, VEGF inhibitors, Multiple myeloma therapies, RAF-MEK inhibitors
-
Left ventricular hypertrophy
- Moderate risk (M1): Multiple myeloma therapies
-
Cardiac amyloidosis
- Very high risk: Multiple myeloma therapies
-
Elevated baseline cTn
- Moderate risk (M1/M2): Anthracycline, HER2 therapies, VEGF inhibitors, Multiple myeloma therapies, RAF-MEK inhibitors
-
Elevated baseline NPs
- Moderate risk (M1/M2): Anthracycline, HER2 therapies, VEGF inhibitors, Multiple myeloma therapies, RAF-MEK inhibitors
- High risk: Multiple myeloma therapies
-
Age ≥80 years
- High risk: Anthracycline, HER2 therapies, RAF-MEK inhibitors
-
Age 65–79 years
- Moderate risk (M2): Anthracycline, HER2 therapies, RAF-MEK inhibitors
-
Age ≥75 years
- High risk: VEGF inhibitors, BCR-ABL inhibitors, Multiple myeloma therapies
-
Age 65–74 years
- Moderate risk (M1/M2): VEGF inhibitors, BCR-ABL inhibitors, Multiple myeloma therapies
-
Age ≥60 years
- Moderate risk (M1): BCR-ABL inhibitors, RAF-MEK inhibitors
-
Cardiovascular disease 10-year risk score >20%
- High risk: BCR-ABL inhibitors
-
Hypertension
- Moderate risk (M1): All treatment types
- High risk: VEGF inhibitors
-
Chronic kidney disease
- Moderate risk (M1): All treatment types
-
Proteinuria
- Moderate risk (M1): VEGF inhibitors
-
Diabetes mellitus
- Moderate risk (M1): All treatment types
-
Hyperlipidaemia
- Moderate risk (M1): VEGF inhibitors, BCR-ABL inhibitors, Multiple myeloma therapies
-
Family history of thrombophilia
- Moderate risk (M1): BCR-ABL inhibitors, Multiple myeloma therapies
-
Dexamethasone >160 mg/month
- Moderate risk (M1): Multiple myeloma therapies
-
Includes anthracycline before HER2-targeted therapy
- Moderate risk (M1g): HER2 therapies
-
Anthracycline (previous exposure)
- High risk: Anthracycline, VEGF inhibitors, Multiple myeloma therapies, RAF-MEK inhibitors
- Moderate risk (M2h): HER2 therapies
-
Trastuzumab (previous exposure)
- Very high risk: HER2 therapies
-
RT to left chest or mediastinum (previous exposure)
- High risk: Anthracycline, Multiple myeloma therapies, RAF-MEK inhibitors
- Moderate risk (M2): HER2 therapies, RAF-MEK inhibitors
- Moderate risk (M1): VEGF inhibitors, Multiple myeloma therapies
-
Non-anthracycline chemotherapy (previous exposure)
- Moderate risk (M1): Anthracycline
-
Lifestyle risk factors
- Current smoker/smoking history: Moderate risk (M1): All treatment types, High risk: BCR-ABL inhibitors
- Obesity (BMI >30 kg/m²): Moderate risk (M1): All treatment types
ABPI: ankle-brachial pressure index; BCR-Abl: breakpoint cluster region-abelson oncogene locus; BMI: body mass index; CABG: coronary artery bypass graft; cTn: cardiac troponin; CTRCD: cancer therapy-related cardiac dysfunction; CV: cardiovascular; CVD: cardiovascular disease; DVT: deep vein thrombosis; HER2: human epidermal receptor 2; HF: heart failure; IMID: immunomodulatory drugs; LV: left ventricular ejection fraction; MEK: mitogen-activated protein kinase; MI: myocardial infarction; NP: natriuretic peptide; PCI: percutaneous coronary intervention; PE: pulmonary embolism; PI: proteasome inhibitors; QTc: QT interval; RAF: proto-oncogene B-RAF; RT: radiotherapy; TKI: tyrosine kinase inhibitor; VEGF: vascular endothelial growth factor inhibitors
Risk level: Low risk = no risk factors OR one moderate1 risk factor; moderate risk (M) = moderate risk factors with a total of 2–4 points (Moderate 1 [M1] = 1 point; Moderate [M2] = 2 points); high risk (H) = moderate risk factors with a total of ≥5 points OR any high-risk factor; very-high risk (VH) = any very-high risk factor.
a AF, atrial flutter, ventricular tachycardia, or ventricular fibrillation.
b Elevated above the ULN of the local laboratory reference range.
c Systolic BP > 140 mmHg or diastolic BP > 90 mmHg, or on treatment.
d eGFR < 60 mL/min/1.73 m2.
e HbA1c > 7.0% or >53 mmol/mol, or on treatment.
f Non-high density lipoprotein cholesterol >3.8 mmol/L (>145 mg/dL) or on treatment.
g High risk if anthracycline chemotherapy and trastuzumab delivered concurrently.
h Previous malignancy (not current treatment protocol).
Table 2 shows the toxicity definition of some of the most common cancer treatments.
Table 2. Cancer therapy-related cardiovascular toxicity definitions.
With permission from [1]. Lyon AR, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43:4229-361.
Cancer Therapy–Related Cardiac Dysfunction (CTRCD)
-
Symptomatic CTRCD (Heart failure)
- Very severe: Requiring inotropic support, mechanical circulatory support, or consideration for transplantation
- Severe: Heart failure hospitalisation
- Moderate: Need for outpatient intensification of diuretic and heart failure therapy
- Mild: Mild heart failure symptoms, no intensification of therapy required
-
Asymptomatic CTRCD
- Severe: New LVEF reduction to <40%
- Moderate: New LVEF reduction by ≥10 percentage points to an LVEF of 40–49%, OR new LVEF reduction by <10 percentage points to 40–49% AND new relative decline in GLS by >15% from baseline and/or new rise in cardiac biomarkers
- Mild: LVEF ≥50% AND new relative decline in GLS by >15% from baseline and/or new rise in cardiac biomarkers
-
Pathohistological diagnosis (EMB)
- Multifocal inflammatory cell infiltrates with overt cardiomyocyte loss by light microscopy
-
Clinical diagnosis
- Cardiac troponin elevation with 1 major criterion or 2 minor criteria
- Major criterion: NMR diagnostic for acute myocarditis
- Minor criteria:
- Clinical syndrome
- Ventricular arrhythmia and/or new conduction system disease
- Decline in left ventricular systolic function, with or without regional wall motion abnormalities (non-Takotsubo pattern)
- Other immune-related adverse events
- Suggestive NMR
- Cardiac troponin elevation with 1 major criterion or 2 minor criteria
-
Severity of myocarditis
- Fulminant
- Non-fulminant: Symptomatic but hemodynamically and electrically stable
- Steroid refractory: Non-resolving or worsening despite high-dose methylprednisolone
-
Recovery from myocarditis
- Complete recovery: Resolution of symptoms, normalization of biomarkers, recovery of LVEF, CMR without acute oedema
- Recovering: Improvement but not normalization, on tapering immunosuppression
-
Asymptomatic vascular toxicity
- Coronary artery disease
- Peripheral arterial disease
- Carotid artery disease
- Venous thrombosis
- Arterial thrombosis
- Peripheral vasoreactivity
- Coronary epicardial vasoreactivity
- Coronary microvascular vasoreactivity
-
Symptomatic vascular toxicity
- Stroke
- Transient ischaemic attack
- Myocardial infarction
- Acute coronary syndromes
- Chronic coronary syndromes
- Peripheral arterial disease
- Vasospastic angina
- Microvascular angina
- Raynaud’s phenomenon
- Arterial hypertension (HTN)
- Cardiac arrhythmias
1.4 Which cancer survivors require long-term cardiovascular surveillance?
The timing of the first cardiology re-evaluation after any cardiotoxic cancer treatment should take into account the level of risk at baseline according to the risk criteria shown in section 1.3.
This assessment at 12 months (and at 3 months in the subjects identified before and during treatment as being at high and very high risk) allows the identification of high-risk patients when they show these characteristics:
- Baseline high risk or very high risk after the evaluation at pre-treatment based on HFA-ICOS criteria
- Cancer therapy with:
- Doxorubicin ≥250 mg/m2
- RT >15 Gy MHD
- Both doxorubicin ≥100 mg/m2 and RT ≥ 5–15Gy MHD
- Haematopoietic stem-cell transplantation
- Moderate or severe CTR-CVT during cancer treatment, especially LV dysfunction, immune checkpoint inhibitor (ICI)-related myocarditis, arrhythmias or severe vascular toxicities.
- New asymptomatic abnormalities in echocardiography and biomarkers, or new CV symptoms at the end of therapy evaluation.
2. Recommendations for the patients and the clinicians at end of cancer therapy
Beyond the risk evaluation of future CTR-CVT, at the end of cancer therapy should be considered other relevant recommendations for all physicians who have in care CS.
In patients with moderate or severe symptomatic or severe asymptomatic CTR-CVT during cancer treatment, HF protective drugs should be continued lifelong.
Selected patients with asymptomatic mild or moderate CTR-CVT, who are taking cardioprotective medications initiated during cancer therapy and who have fully recovered with normal echocardiography and cardiac biomarkers may be eligible to be weaned off CV therapy.
The end of therapy evaluation may also include the execution of a cardiopulmonary exercise test (CPET) for those CS experiencing exertional limitations and who may benefit from cardiac rehabilitation. Notably, patients submitted to higher doses of anthracycline or RT, with high CV risk at baseline or with abnormalities in LV function during or at the end of therapy, should undergo a CPET as this may identify CV versus non-CV causes of symptoms.
Other recommendations may be summarised as follows:
- Education and support of CS to adopt appropriate healthy lifestyle measures
- Periodical assessment of common CV risk factors
- Education of CS regarding recognition for early signs and symptoms of CTR-CVT
- Prompt cardiology referral of CS with new symptoms or new asymptomatic abnormalities in echocardiography and cardiac biomarkers
- Appropriate cardiac rehabilitation in CS with high CV risk
Moreover, specific categories of patients require specific interventions:
- The development of hypertension during cancer therapy with TKI requires a CV follow-up and treatment optimisation
- Patients who develop vascular toxicities during cancer therapy need specific management measures
- The occurrence of a prolongation of the QT or true long QT syndrome (LQTS) after certain chemotherapy agents indicates a regular ECG follow-up
Note to editors
Author:
Riccardo Asteggiano MD, FESC, Adjunct Professor
Department of Medicine and Surgery, University of Insubria, Varese, Italy;
LARC-Laboratorio Analisi e Ricerca Clinica, Turin, Italy
Address for correspondence:
Dr Riccardo Asteggiano, Via San Marino 11 a, 10134 Turin, Italy
Author disclosures:
The author has no conflicts of interest to declare with regards this article.
References
- Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, Cutter DJ, de Azambuja E, de Boer RA, Dent SF, Farmakis D, Gevaert SA, Gorog DA, Herrmann J, Lenihan D, Moslehi J, Moura B, Salinger SS, Stephens R, Suter TM, Szmit S, Tamargo J, Thavendiranathan P, Tocchetti CG, van der Meer P, van der Pal HJH; ESC Scientific Document Group, Lancellotti P, Thuny F, Abdelhamid M, Aboyans V, Aleman B, Alexandre J, Barac A, Borger MA, Casado-Arroyo R, Cautela J, Čelutkienė J, Cikes M, Cohen-Solal A, Dhiman K, Ederhy S, Edvardsen T, Fauchier L, Fradley M, Grapsa J, Halvorsen S, Heuser M, Humbert M, Jaarsma T, Kahan T, Konradi A, Koskinas KC, Kotecha D, Ky B, Landmesser U, Lewis BS, Linhart A, Lip GYH, Løchen ML, Malaczynska-Rajpold K, Metra M, Mindham R, Moonen M, Neilan TG, Nielsen JC, Petronio AS, Prescott E, Rakisheva A, Salem JE, Savarese G, Sitges M, Ten Berg J, Touyz RM, Tycinska A, Wilhelm M, Zamorano JL. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS).Eur Heart J. 2022;43:4229-361.
- Lyon AR, Dent S, Stanway S, Earl H, Brezden-Masley C, Cohen-Solal A, Tocchetti CG, Moslehi JJ, Groarke JD, Bergler-Klein J, Khoo V, Tan LL, Anker MS, von Haehling S, Maack C, Pudil R, Barac A, Thavendiranathan P, Ky B, Neilan TG, Belenkov Y, Rosen SD, Iakobishvili Z, Sverdlov AL, Hajjar LA, Macedo AVS, Manisty C, Ciardiello F, Farmakis D, de Boer RA, Skouri H, Suter TM, Cardinale D, Witteles RM, Fradley MG, Herrmann J, Cornell RF, Wechelaker A, Mauro MJ, Milojkovic D, de Lavallade H, Ruschitzka F, Coats AJS, Seferovic PM, Chioncel O, Thum T, Bauersachs J, Andres MS, Wright DJ, López-Fernández T, Plummer C, Lenihan D. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society.Eur J Heart Fail. 2020;22:1945-60.
- Čelutkienė J, Pudil R, López-Fernández T, Grapsa J, Nihoyannopoulos P, Bergler-Klein J, Cohen-Solal A, Farmakis D, Tocchetti CG, von Haehling S, Barberis V, Flachskampf FA, Čeponienė I, Haegler-Laube E, Suter T, Lapinskas T, Prasad S, de Boer RA, Wechalekar K, Anker MS, Iakobishvili Z, Bucciarelli-Ducci C, Schulz-Menger J, Cosyns B, Gaemperli O, Belenkov Y, Hulot JS, Galderisi M, Lancellotti P, Bax J, Marwick TH, Chioncel O, Jaarsma T, Mullens W, Piepoli M, Thum T, Heymans S, Mueller C, Moura B, Ruschitzka F, Zamorano JL, Rosano G, Coats AJS, Asteggiano R, Seferovic P, Edvardsen T, Lyon AR. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: a position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC).Eur J Heart Fail. 2020;22:1504-24.
- Pudil R, Mueller C, Čelutkienė J, Henriksen PA, Lenihan D, Dent S, Barac A, Stanway S, Moslehi J, Suter TM, Ky B, Štěrba M, Cardinale D, Cohen-Solal A, Tocchetti CG, Farmakis D, Bergler-Klein J, Anker MS, Von Haehling S, Belenkov Y, Iakobishvili Z, Maack C, Ciardiello F, Ruschitzka F, Coats AJS, Seferovic P, Lainscak M, Piepoli MF, Chioncel O, Bax J, Hulot JS, Skouri H, Hägler-Laube ES, Asteggiano R, Fernandez TL, de Boer RA, Lyon AR. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: a position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology.Eur J Heart Fail. 2020;22:1966-83.
- Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy.Circulation. 2015;131:1981-8.
- Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida JM, Capodanno D, Cosyns B, Crawford C, Davos CH, Desormais I, Di Angelantonio E, Franco OH, Halvorsen S, Hobbs FDR, Hollander M, Jankowska EA, Michal M, Sacco S, Sattar N, Tokgozoglu L, Tonstad S, Tsioufis KP, van Dis I, van Gelder IC, Wanner C, Williams B; ESC Scientific Document Group. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice.Eur J Prev Cardiol. 2022;29:5-115.
















