The Revolution in Pharmacotherapy: From Herbs to Pills to Antibodies and Nucleic Acids

Objectives of the meeting
Historical Development of Pharmacotherapy
Pharmacotherapy started with herbs that were sometimes useful, but often toxic. Then we moved to pills, where the active ingredient (for example for digitalis, the steroid) was purified and a more precise dosage and avoidance of side-effects, as much as possible, became a reality. Pharmacotherapy today relies on pills containing a vast number of molecules interfering with cell surface receptors (e.g. a/b-receptors, AT1-receptors, P2Y12-receptors), enzymes (ACE, neprilysin), ion/metabolite transporters (SGLT2) and other pathways for the treatment of blood pressure, lipids, diabetes, coronary artery disease and heart failure. More recently, antibodies interfering with crucial proteins have become clinical practice, after rheumatology (infliximab, adalimumab, among others) now also in cardiology with antibodies against PCSK9.
Final Programme
DAY 1: 31 January 2024 - 14:00-18:15
- 12:30-14:00 - Arrival and buffet lunch
- 14:00-14:05 - Welcome from the CRT Chairpersons - Thomas F. Lüscher, Alexandra Goncalves (BMS)
- 14:05-14:15 - General introduction to the workshop - Ulf Landmesser, Thomas F. Lüscher
- 14:15-18:30 - SESSION 1 – NucleIC Acids: Concept and Potential (Moderated by: Francesco Cosentino and Alexandra Goncalves (BMS))
-
14:15-14:30 - From Herbs to pills, antibodies and nucleic acids: The rise of pharmacotherapy - Thomas F. Lüscher
-
14:30-14:50 - Efficacy and safety of siRNA and ASO therapeutics - Ulf Landmesser
-
14:50-15:10 - The future of nucleic acid therapeutics: The industries view - Christopher O’Donnell (Novartis)
-
15:10-15:35 - Nucleic acid-based therapeutics for CVDs: safety insights from EMA Scientific Advice and marketing authorizations - Gabriella Passacquale, Anna Tavridou, and Clemens Mittmann (EMA) via Zoom
-
15:35-15:50 - Coffee break
-
15:50-16:50 - BREAKOUT SESSIONS 1: The future of nucleoid acids - 60 min
- Group 1: Ethics - Lead: Julian März, Rapporteur: Jürgen Prochaska (BI)
- Group 2: Safety and Regulatory approval - Lead: Ulf Landmesser, Rapporteur: Paul Nioi (Alnynam)
- Group 3: Future perspectives from the medical side - Lead: Francesco Cosentino, Rapporteur: Anders Himmelmann (AstraZeneca)
-
17:00-17:10 - Report from breakout session 1 – 10 mins - Rapporteur: Jürgen Prochaska (BI)
-
17:10-17:20 - Report from breakout session 2 – 10 mins - Rapporteur: Paul Nioi (Alnynam)
-
17:20-17:30 - Report from breakout session 3 – 10 mins - Rapporteur: Anders Himmelmann (AstraZeneca)
-
17:30-18:00 - How do we implement new therapeutics to change the paradigm? - Industry’s view: Andres Laguna (Novartis), Academic’s view: Lale Tokgözoğlu
-
18:00-18:20 - Wrap-up and summary Day 1 – Outlook to Day 2 - Chairpersons
-
END OF DAY 1
-
20:00 - Dinner
DAY 2: 01 February 2024 - 08:00 - 12:45
-
08:00 - 12:45 - SESSION 2 – From Antibodies to Nucleotid Acids and beyond (Moderated by: Ulf Landmesser and Jürgen Prochaska (Boehringer Ingelheim Pharmaceuticals Inc))
-
08:00-08:30 - Cardiovascular Gene Therapy - Efficient gene delivery to the heart? What are the best targets? - Roger Hajjar
-
08:30-09:00 - ASO/siRNa vs. antibodies for TTR amyloid heart disease - Mathew Maurer
-
09:00-09:30 - miRNAs as therapeutic tools - Thomas Thum
-
09:30-10:00 - CRISPR-Cas9: From treatment to cure? - Sekar Kathiresan (Verve Therapeutics)
-
10:00-10:15 - Coffee break
-
10:15-11:15 - BREAKOUT SESSIONS 2: From Treatment to cure - 60 min
- Group 1: Compliance with the new tools - Lead: Francesco Cosentino, Rapporteur: Ricardo Rocha (Intellia Therapeutics)
- Group 2: miRNAs - Lead: Christopher O’Donnell (Novartis), Rapporteur: Tomasz Guzik
- Group 3: How to target the heart or the kidney: in search of novel strategies - Lead: Benjamin Meder, Rapporteur: Lothar Roessig (ASKBIO/Bayer)
-
11:15-11:25 - Report from breakout session 1 – 10 mins - Rapporteur: Ricardo Rocha (Intellia Therapeutics)
-
11:25-11:35 - Report from breakout session 2 – 10 mins - Rapporteur: Tomasz Guzik
-
11:35-11:45 - Report from breakout session 3 – 10 mins - Rapporteur: Lothar Roessig (ASKBIO/Bayer)
-
11:45-12:05 - Patient’s perspectives on nucleoid acid therapy - Mattias Van Heetvelde (ESC Patient Forum)
-
12:05-12:35 - Ethical issues of genetic therapeutic tools - Julian März
-
12:35-12:45 - Wrap-up, conclusions, next steps for a publication - Chairpersons
-
END OF DAY 2
-
13:00 - Buffet lunch and departures
Nucleotid acids as novel therapeutics
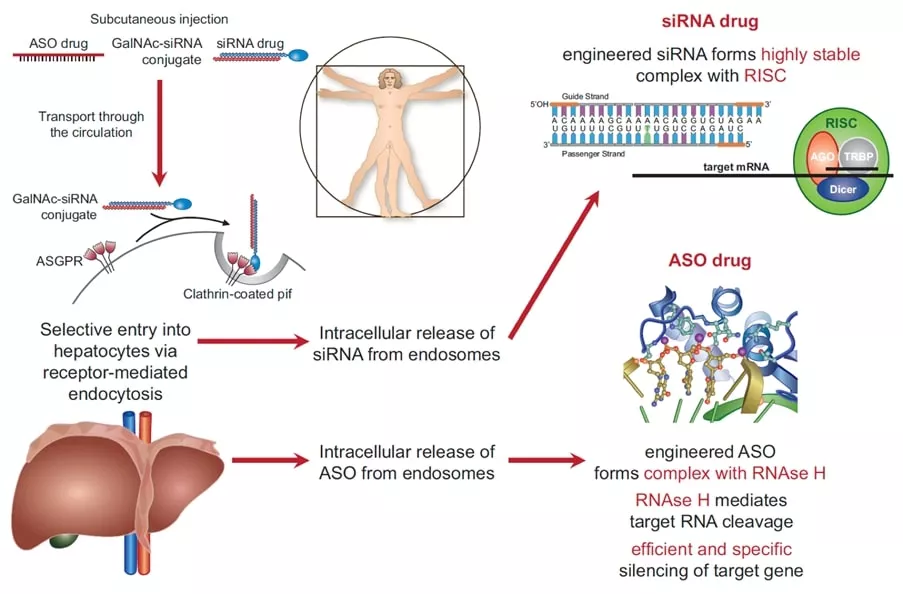
A true revolution of pharmacotherapy took place with the discovery of RNA interference by Andrew Fire and Craig Mello, Nobel Prize Laureates in 2006, as well as the use of antisense oligonucleotides. These molecules no longer interfere with surface receptors or enzymes in the circulation or in cardiovascular tissue, but rather prevent the translation of messenger RNA into a target protein (Figure 1). When combined with tags interfering with specific surface receptors (e.g. GalNac with Asialoglycoprotein receptors on hepatocytes) these nucleic acids can be made highly organ and tissue specific and often have a long duration of action (up to >6 months).
CRISPR-Cas9: From treatment to cure

Of note, the future holds even more promising tools; indeed, with the discovery of the gene scissor, CRISPR-Cas9, by Emmanuelle Charpentier and Jennifer Doudna, Nobel Laureates in 2020 (Figure 2) gene editing has become possible with a single treatment in a lifetime for pa-tients with various medical conditions. Indeed, in Amyloidosis already a few patients have been successfully treated and as for lipids, the technique has been successfully used in primates.
These new developments in pharmacotherapy bring enormous opportunities, but also questions as regards their safety and sustainability, but they will certainly change medicine as it has been practiced in the past.
Objectives of the CRT meeting: To discuss the basics of this new pharmacotherapy, its current application and future perspectives, as well as important safety and ethical issues associated with it. Furthermore, the goal is to involve the European Medical Agency (and possibly the Federal Drug Administration in the USA) to discuss registration issues with these new technologies, and in particular, as regards the advent of CRISPR-Cas9.
Related Reading

From traditional pharmacological towards nucleic acid-based therapies for cardiovascular diseases
Session Recording
Day 1
Day 2
Academic Chairpersons

Professor Thomas Felix Luescher
ESC President-elect and ESC Chair of the CRT
Industry Chairpersons
Doctor Andres Laguna Fernandez
Novartis
Professor Juergen Prochaska
Boehringer Ingelheim Pharmaceuticals Inc
Doctor Ricardo Rocha
Intellia Therapeutics

Doctor Alexandra Goncalves
BMS (Industry Chair of the CRT)
Biographies
















