DYSLIPIDAEMIA. DEDICATED TO SCOTT GRUNDY, A PIONEER IN LIPIDOLOGY
Keywords
bempedoic acid, statin-intolerant patients, cholesterol, lipid-lowering agents
Take-home messages
- Bempedoic acid is an oral ACLY inhibitor activated in the liver (va ACSVL1), reducing cholesterol synthesis upstream of HMG-CoA reductase; limited activation in muscle helps avoid myotoxicity.
- BA is an effective and safe lipid lowering therapy with a hard endpoints trial.
- CLEAR Outcomes demonstrates a measurable reduction in major CV events (≈13% relative) in statin-intolerant high-risk patients over ~3.4 years, supporting use in those populations.
- Safety profile is acceptable; increased gout, hyperuricaemia, and cholelithiasis; muscle symptoms not materially increased.
- 2025 ESC/EAS guidelines have upgraded BA to Class I recommendation for statin intolerance, Class IIa for add-on to statin when goals unmet.
- In practice, BA is not a substitute for high-intensity statin in those who tolerate statins, but provides an additional tool, especially when statin therapy is limited.
Introduction
Lowering low-density lipoprotein cholesterol (LDL-C) remains foundational in reducing atherosclerotic cardiovascular disease (ASCVD). Statins, ezfetimibe, and PCSK9 inhibitors have established roles. Bempedoic acid (BA) is an oral ATP‐citrate lyase (ACLY) inhibitor, relatively recently added to the lipid-lowering armamentarium. In statin-intolerant or statin-suboptimal patients, plus in combination therapy, it has shown promise in both LDL-C reduction and in reducing cardiovascular (CV) events. With the 2025 ESC/EAS-focused update, BA has gained stronger guideline endorsement. This review surveys its mechanism, randomised trial data with hard endpoints, safety, and role per the latest guidelines, with implications for practice.
Mechanism of action and pharmacology
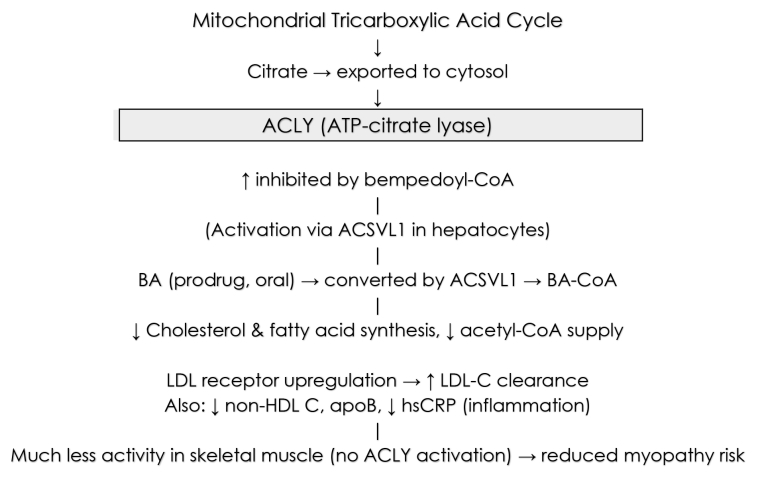
Bempedoic acid (BA; also known as ETC-1002) is an orally administered prodrug converted in hepatocytes by very-long-chain acyl-CoA synthetase-1 (ACSVL1, encoded by SLC27A2) to its active coenzyme-A derivative (bempedoyl-CoA). This active moiety inhibits ATP-citrate lyase (ACLY), a cytosolic enzyme that converts citrate (exported from mitochondria) to acetyl-CoA, a key substrate for cholesterol and fatty-acid synthesis. By acting upstream of HMG-CoA reductase (the target of statins), BA reduces cholesterol flux, upregulates low-density lipoprotein (LDL) receptor expression, and lowers LDL cholesterol (LDL-C). Because ACSVL1 has minimal expression in skeletal muscle, activation of BA (and thus ACLY inhibition) is largely confined to the liver, reducing risk of muscle toxicity. Additionally, BA has been shown to reduce systemic inflammation (e.g., high sensitivity C-reactive protein, [hsCRP]). Some ancillary metabolic effects have been reported (fatty acid synthesis, possibly effects on gluconeogenesis or glucose metabolism), though these are less well established in outcomes. Pharmacokinetically, the dose is 180 mg once daily, cleared mainly via glucuronidation and renal routes; it has a moderate half-life and few significant interactions beyond statin potentiation via organic anion transporter polyeptide-2 (OATP2 [and other transporters]) and effects on uric acid [1].
Central Figure. Mechanism of activation and action of bempedoic acid.
ACLY: ATP-citrate lyase; ACSVL1: very-long-chain acyl-CoA synthetase-1; BA: bempedoic acid; hsCRP: high sensitivity C reactive protein; apoB: apolipoprotein B
Phase 3 trials: LDL-C reduction and safety
Before outcome data, several phase 3 randomised controlled trials (RCTs) evaluated LDL-C lowering, safety, and tolerability in various populations: statin-intolerant, on maximally tolerated statin, or needing further LDL-C reduction. (2-5)
Table 1. Bempedoic acid studies.
Trial: CLEAR Harmony [2] (ASCVD/HeFH pts, maximally tolerated statin)
- Population
- High risk; ASCVD or heterozygous familial hypercholesterolaemia
- Background therapy
- Statin therapy (maximally tolerated) ± other LLT
- Baseline LDL-C (approximate)
- ~100–110 mg/dL
- Placebo-corrected LDL-C reduction at 12 weeks
- ~18-19% reduction BA vs placebo
- Other lipid/ inflammatory effects
- Non-HDL-C, total cholesterol, apoB lowered; hsCRP modestly reduced
- Key safety/tolerability observations
- Adverse events similar overall; slightly more discontinuation; muscle symptoms similar to placebo
Trial: CLEAR Wisdom [3]
- Population
- High-risk ASCVD/HeFH, LDL-C ≥70 mg/dL, on statins or LLT
- Background therapy
- Maximally tolerated statin therapy
- Baseline LDL-C (approximate)
- ~120 mg/dL
- Placebo-corrected LDL-C reduction at 12 weeks
- Placebo-corrected ~17.4% at Week 12
- Other lipid/ inflammatory effects
- Reductions in non-HDL-C, apoB; median hsCRP ~18-20% down
- Key safety/tolerability observations
- Hyperuricaemia more common; other AE similar; small increased UTI, etc
Trial: CLEAR Serenity [4]
- Population
- Statin intolerant pts
- Background therapy
- Minimal or no statin use
- Baseline LDL-C (approximate)
- ≥130 mg/dL (or ≥100 if HeFH)
- Placebo-corrected LDL-C reduction at 12 weeks
- ~21% reduction at 12 weeks; similar at 24 weeks
- Other lipid/ inflammatory effects
- hsCRP lowered (~20–25%); non-HDL, apoB similarly lowered
- Key safety/tolerability observations
- Muscle symptoms numerically lower vs placebo; gout more frequent; more discontinuations due to AEs in BA arm
Trial: CLEAR Tranquility [5]
- Population
- Statin intolerant, needing additional LDL-C lowering
- Background therapy
- BA + ezetimibe vs ezetimibe ± low statin
- Baseline LDL-C (approximate)
- ≥100 mg/dL
- Placebo-corrected LDL-C reduction at 12 weeks
- ~28.5% reduction at 12 weeks (BA + ezetimibe vs ezetimibe alone)
- Other lipid/ inflammatory effects
- Non-HDL, apoB reduced; hsCRP about one-third (≈33%) drop
- Key safety/tolerability observations
- Safety consistent; no large muscle AE signal; increased uric acid etc
AE: adverse events; apoB: apolipoprotein B; ASCVD: atherosclerotic cardiovascular disease; BA: bempedoic acid; HDL-C: high-density lipoprotein cholesterol; HeFH, heterozygous familial hypercholesterolaemia; hsCRP: high sensitivity C-reactive protein; LDL-C, lipoprotein cholesterol; LLT, lipid lowering therapy; UTI: urinary tract infection
These trials showed that BA achieves moderate LDL-C lowering (15-30%) depending on baseline, background therapy, or combination with other medications (e.g., with ezetimibe). Reductions in non-high density lipoprotein cholesterol (HDL-C), apolipoprotein B (apoB) and hsCRP are consistent and clinically relevant. Tolerability is generally acceptable; statin-intolerant populations especially benefit from an option without muscle toxicity.
CLEAR Outcomes: cardiovascular outcomes and hard endpoints
The landmark outcome data comes from CLEAR Outcomes:
Key features and findings:
Design/population: Randomised, double-blind, placebo-controlled trial of 13,970 statin‐intolerant adults with established ASCVD or at high risk for ASCVD. Baseline LDL-C ≈139 mg/dL. Median follow-up ~40.6 months. Patients were statin-intolerant or unwilling to receive statins. Part of them (23%) were receiving very low doses of statin (an average daily dose of rovustatin <5 mg, atorvastatin <10 mg, simvastatin <10 mg, lovastatin <20 mg, pravastatin <40 mg, fluvastatin <40, or pitavastatin <2 mg). [6]
Primary endpoint: 4-component major adverse cardiovascular events (MACE-4) — CV death, non-fatal myocardial infarction (MI), non-fatal stroke, or coronary revascularisation.
Results:
- Primary event rate: 11.7% in BA versus 13.3% in placebo; hazard ratio (HR) 0.87 (95% confidence interval [CI]: 0.79-0.96; p=0.004). Absolute risk reduction ~1.6%.
- 3-component MACE (CV death, non-fatal MI, non-fatal stroke) also significantly reduced. Fatal/non-fatal MI reduced; stroke reduction not statistically significant.
- LDL-C lowering: ~21% versus near-zero in placebo at 6 months; hsCRP reduced (~20%) at 12 months.
Subgroup/secondary analyses:
- Glycaemic status: Patients with diabetes had significant relative and absolute event reduction; no evidence of effect modification across baseline glycaemia (normoglycaemia, prediabetes, diabetes).
- Obesity subset (body mass index ≥30 kg/m2): LDL-C reduction ~22.5%, hsCRP ~23.2%; MACE-4 reduced by ~23% (HR 0.77) versus placebo; MI, revascularisation, stroke also reduced.
Total event analysis:
BA reduced not only the first events but also total CV events (including recurrent) versus placebo.
• Mortality: No significant difference in all-cause mortality or CV mortality in the main trial.
Hence, CLEAR Outcomes demonstrates that in statin-intolerant, high-risk patients, BA yields a modest but statistically significant relative risk reduction (~13% for primary composite), with benefit proportionally increasing with higher risk and more events, correlated with the degree of LDL-C lowering.
Safety, adverse events, and metabolic effects
Major adverse and notable side effects
- Hyperuricaemia and gout: In CLEAR Outcomes, hyperuricaemia occurred in ~10.9% BA versus 5.6% placebo; gout 3.1% versus 2.1%.
- Cholelithiasis (gallstones): Slightly higher in BA (≈2.2%) versus placebo (≈1.2%).
- Muscle symptoms: Despite the mechanistic rationale of low muscle activation, in trials in statin-intolerant patients, some muscle symptoms were reported. However, incidence was similar or even somewhat lower versus placebo in some settings. There was no large increase in myopathy or rhabdomyolysis.
- Renal/creatinine/hepatic enzymes: Mild increases in serum creatinine and possible renal events in some subgroup analyses; some small hepatic enzyme elevations. Generally, clinically tolerable.
- Discontinuations: Slightly higher discontinuation for adverse events in BA in some trials.
Metabolic effects
- Glucose/diabetes: In the glycaemic status prespecified analysis of CLEAR Outcomes, patients with diabetes had significant reduction of the primary endpoint; in those without, BA did not significantly alter risk of new-onset diabetes. Haemoglobin A1c and fasting glucose were not affected by BA.
- Inflammation: Consistent reductions in hsCRP (~15-25%) in phase 3 trials, and in CLEAR Outcomes (~20%) over 12 months.
Comparative efficacy: how much LDL-C lowering according to situation
Some quantitative benchmarks help to place BA relative to other therapies:
- As an add-on to maximally tolerated statin therapy, BA yields around 15-20% LDL-C lowering at ~12 weeks (CLEAR Wisdom, Harmony).
- In statin-intolerant patients (monotherapy or minimal statin), BA may yield LDL‐C reductions of ~20-25%.
- In combination with ezetimibe in statin-intolerant patients, LDL-C reductions ~35%.
These reductions are less than PCSK9 inhibitors, but substantial in settings where statins are not tolerated or limited, or where cost, access, or preference limits injectables. Also, the reduction in non-HDL-C, apoB, and inflammatory markers adds to benefit.
Guidelines and positioning in 2025
The 2025 Focused Update of the ESC/EAS Guidelines [7] for the Management of Dyslipidaemias includes updated recommendations for non-statin therapies, including BA.
Key features:
- Non-statin therapies with proven cardiovascular benefit (including BA) are recommended in patients unable to take statin therapy to lower LDL-C levels and reduce risk of CV events. Class I, Level A.
- Bempedoic acid is specifically recommended in patients who cannot take statin therapy to achieve LDL-C goal. Class I, Level B.
- Addition of BA to maximally tolerated statin (with or without ezetimibe) should be considered (Class IIa, Level C) in patients at high or very high risk who are not at LDL-C goal.
- Also, the guidelines reaffirm the risk stratification framework (Systematic COronary Risk Evaluation [SCORE2] / SCORE2-Older Persons [SCORE2-OP]), LDL-C goals unchanged from 2019 for high and very high risk: e.g., <1.4 mmol/L (<55 mg/dL) for very high risk, <1.8 mmol/L (<70 mg/dL) for high risk. In extreme risk (recurrent events despite maximal therapy), LDL-C <1.0 mmol/L (≈40 mg/dL) may be considered.
Integrating BA into clinical practice: where, when, whom?
From evidence and guidelines, some practical points:
- Statin intolerance/contraindication: BA is especially useful for patients who truly cannot tolerate statins (multiple statins, muscle symptoms, etc). In this population, BA offers both LDL-C lowering and event reduction (CLEAR Outcomes).
- Patients on statins but not at goal: For those already on maximally tolerated statin plus perhaps ezetimibe, yet still with LDL-C above target, BA is an option (especially if patient prefers oral therapy or cannot access injectables).
- Combination with ezetimibe: Use of BA plus ezetimibe improves LDL-C reduction compared to BA alone or ezetimibe alone.
- Patient risk factors: Benefit is greater in those with higher baseline LDL-C, with higher overall vascular risk (e.g., presence of ASCVD, diabetes). Also, obesity subgroup shows similar or even greater relative benefit.
- Safety monitoring: Check uric acid if there is a history of gout; monitor renal function, hepatic enzymes; be alert for gallbladder disease; counsel regarding possible gastro-intestinal effects; watch for drug interactions, especially with statins.
Limitations and uncertainties
- Absolute risk reductions modest: The ~1.6% absolute risk reduction seen in the primary endpoint over ~3.4 years in CLEAR Outcomes means high number needed to treat (NNT) especially if baseline risk is moderate.
- Mortality benefit unclear: No significant reductions in all‐cause or CV mortality in the main trial.
- Longer follow-up especially in lower risk or if primary prevention is needed: The bulk of outcome data is in statin-intolerant or higher risk cohorts; whether BA would benefit broader populations similarly is not fully proven.
- Adherence & real-world tolerance: Clinical trial populations selected, close monitoring; real-world may see more discontinuations or issues.
Synthesis: suggestions for use
Based on mechanism, trial data, and guideline positioning, the following algorithm may help in clinic:
- Assess risk and LDL-C level: determine whether patient is “very high”, “high”, etc, per ESC/EAS 2025; set LDL-C target.
- Initiate statin therapy: highest tolerated dose. If statin intolerance, move to BA (monotherapy or in combination).
- If statin therapy is tolerated but insufficient: add ezetimibe.
- If still above LDL-C goal: consider adding BA (if not already in use) especially if patient prefers oral therapy or if there are PCSK9 inhibitor cost or access issues; consider PCSK9 inhibitors if needed and if feasible for larger LDL-C reduction.
- Monitor safety: uric acid, gout history; hepatic/renal; muscle symptoms (though lower probability); enforce lifestyle.
- Timeline of follow-up: check LDL-C about 4-6 weeks after therapy change; ensure adherence; adjust according to response.