
Breakdown of the Initiative
The project will be achieved through a unique collaboration between medical associations, regulatory agencies, notified bodies, academic institutions, patients’ groups, and health technology assessment agencies.
The consortium is led by the European Society of Cardiology, in close partnership with the European Federation of National Associations of Orthopaedics and Traumatology and involves all 35 specialist medical associations that are members of the Biomedical Alliance in Europe. Final recommendations will be submitted to the European Commission's Working Group on Clinical Investigation and Evaluation for consideration when developing EU guidance or common specifications.
Kick-Off Meeting Press Release
Work Packages
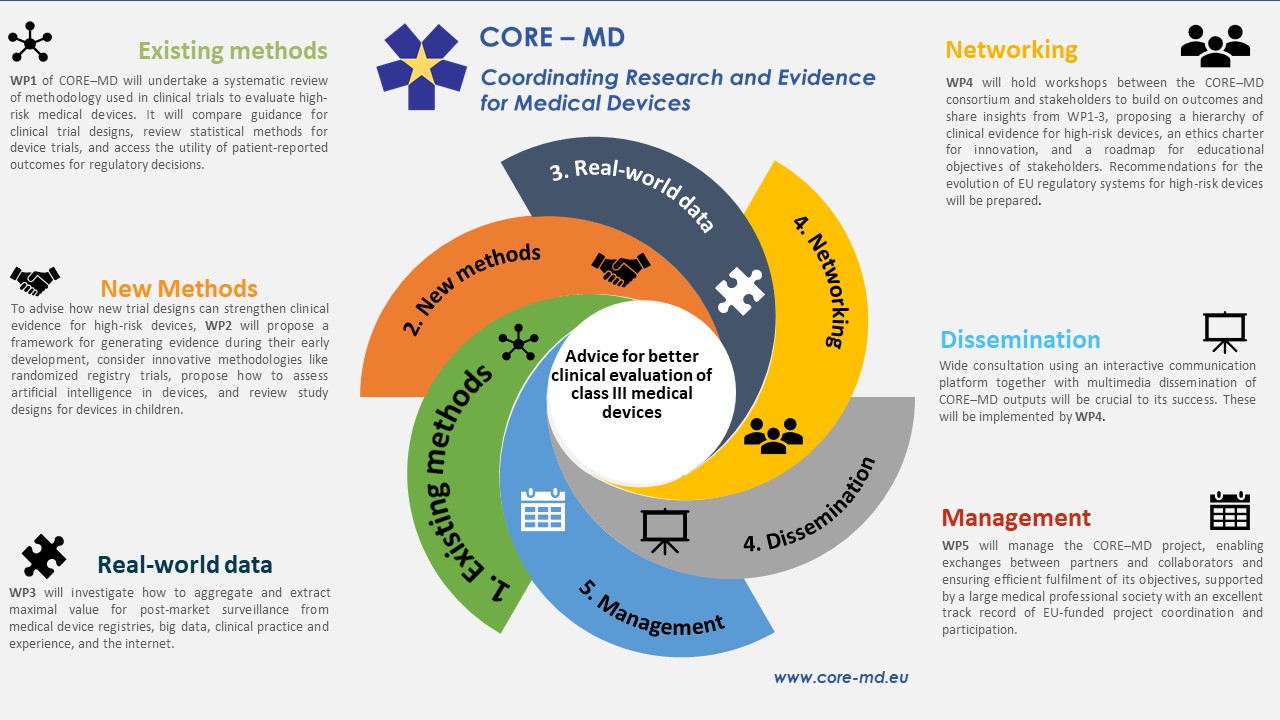
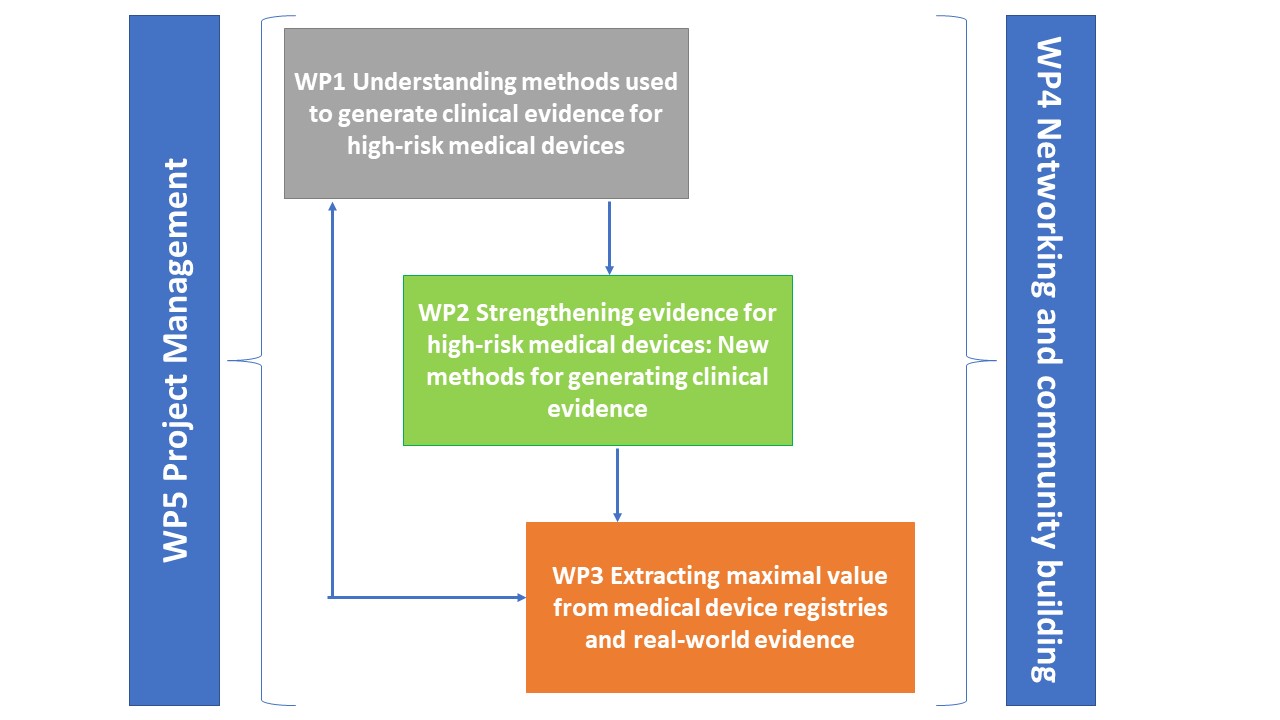
The CORE-MD Project consists of five Work Packages.
Work Package 1 aims at understanding the methods that have been used to generate clinical evidence for high-risk medical devices.
Work Package 2 aims at strengthening the clinical evidence for high-risk medical devices by exploring new methods for generating data about their performance.
The main objective of Work Package 3 is to extract maximal value from medical device registries and real-world evidence.
Work Package 4 and Work Package 5 will focus respectively on engagement with stakeholders, and project management.
Partners in the CORE-MD Consortium
Société Eurpéenne de Cardiologie / European Society of Cardiology
European Federation of the National Associations of Orthopaedics and Traumatology (EFORT)
European Academy of Paediatrics
Academisch Ziekenhuis Leiden (Leiden University Medical Center)
The Chancellor, Masters and Scholars of the University of Oxford
Royal College of Surgeons in Ireland
Insel Gruppe Ag (University Hospital Bern)
Katholieke Universiteit Leuven
UMIT - Private Universität für Gesundheitswissenschaften, Medizinischeinformatik und Technik GmbH (University for Health Sciences, Medical Informatics and Technology, Hall in Tirol, Austria)
Göteborgs Universitet (Gothenburg University)
Politecnico di Milano (Polytechnic University of Milan)
Health Products Regulatory Authority (Dublin)
Laegemiddelstyrelsen (Danish Medicines Agency, Copenhagen)
Urzad Rejestracji Produktow Leczniczych, Wyrobow Medzycznych i Produktow Biobojczych (Office for Registration of Medicinal Products, Medical Devices and Biocidal Products, Warsaw)
Rijksinstituut Voor Volksgezondheid en Milieu (National Institute for Public Health and the Environment, Bilthoven, the Netherlands)
Istituto Superiore di Sanita (Italian National Institute of Health, Rome)
HTA Austria - Austrian Institute for Health Technology Assessment GmbH (Vienna)
Fundacion Publica Andaluza Progreso y Salud (Progress and Health Foundation, Sevilla)
European Association of Notified Bodies for Medical Devices
Contact
Prof. Alan Fraser
CORE-MD Scientific Coordinator
European Society of Cardiology
Brussels, Belgium
Project Website
The website of the project (www.core-md.eu) is under preparation.
Funding Acknowledgement
 The project has received funding from the European Union's Horizon 2020 Research and Innovation Programme under grant agreement No. 965246
The project has received funding from the European Union's Horizon 2020 Research and Innovation Programme under grant agreement No. 965246

 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.