About the initiative
Launched in March 2017, BigData@Heart is a five-year project of the Innovative Medicines Initiative (IMI), an EU public-private consortium consisting of patient networks, learned societies, SMEs, pharmaceutical companies and academia.
The ESC, in partnership with a number of European academic research groups and pharmaceutical companies, have joined forces to develop a big data-driven translational research platform. BigData@Heart has access to most of the relevant large-scale European databases, ranging from EHR and disease registries to well-phenotyped clinical trials and large epidemiological cohorts enriched with –omics data, including data on more than five million patients with acute coronary syndromes, atrial fibrillation, and heart failure and about 20 million controls without the diseases. By accessing and harmonising European-wide data sets, the ambition is to design algorithms that predict the evolution of disease, based on medical history, hospital records, and country-specific statistics.
Using all available data across data modalities, combined with machine learning or Bayesian network models, is expected to further refine outcome prediction. In addition, BigData@Heart will explore and set standards for the use of large and heterogeneously distributed data sets. Investigations include data mapping using common standards, federated data analysis obviating the need for central databases, and the legal and ethical aspects of using consented and unconsented data, in view of the EU general data protection regulation but also across countries with varying privacy rules.
Mapping real-world data sources
The data sweep provides an overview of existing real-world data as a foundation for the identification of potential data sources to conduct observational research studies. The aim of the data sweep is to foster collaboration between researchers, increase access and use of real-world data to strengthen the evidence available, and eventually improve outcomes for patients with heart failure, acute coronary syndrome and atrial fibrillation.
Explaining the approach to collecting data
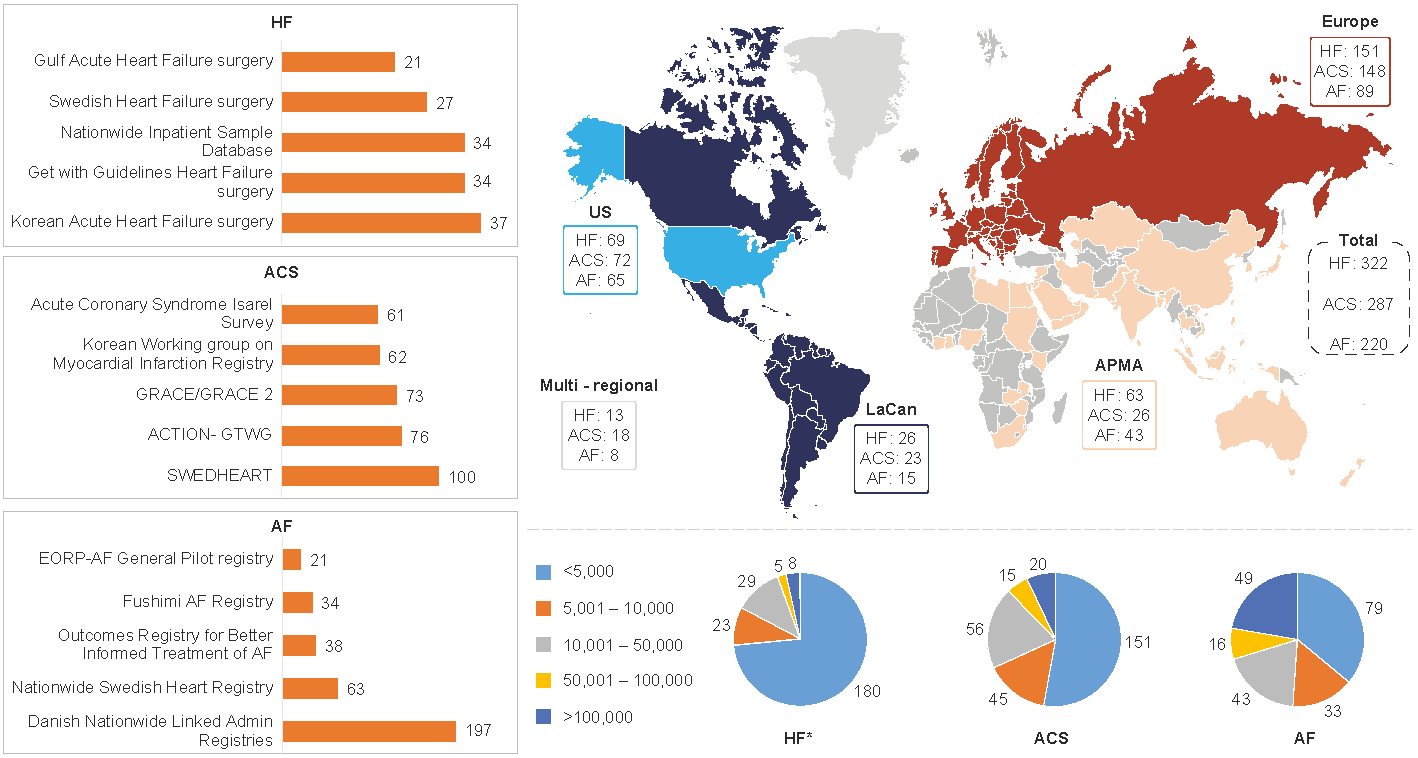
The BigData@Heart consortium conducted a systematic review of publications with global real-world data (RWD) pertaining to heart failure (HF), acute coronary syndrome (ACS) and atrial fibrillation (AF), generating a list of unique data sources.
Metadata were extracted based on the source type (e.g., electronic health records, genomics, clinical data), study design, population size, clinical characteristics, follow-up duration, outcomes and assessment of data available for future studies and linkage. A total of 11,889 publications were retrieved for HF, 10,729 for ACS and 6,262 for AF.
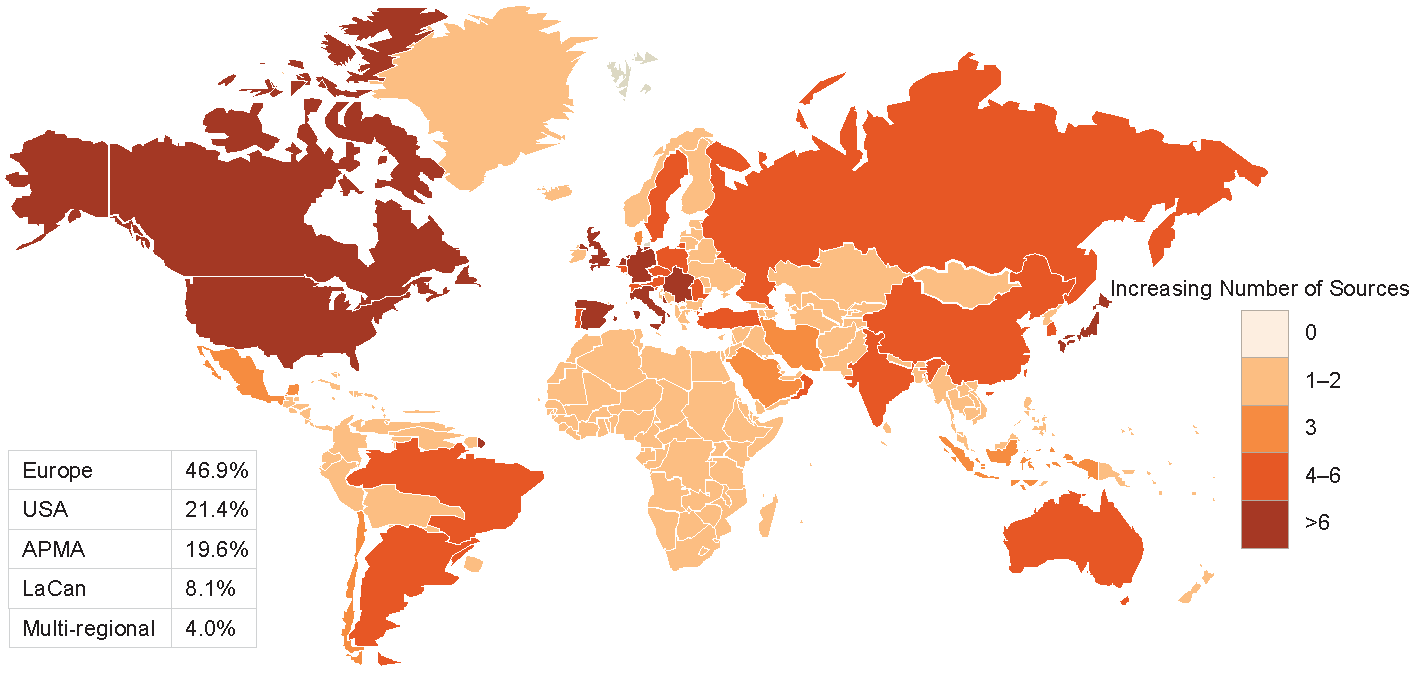
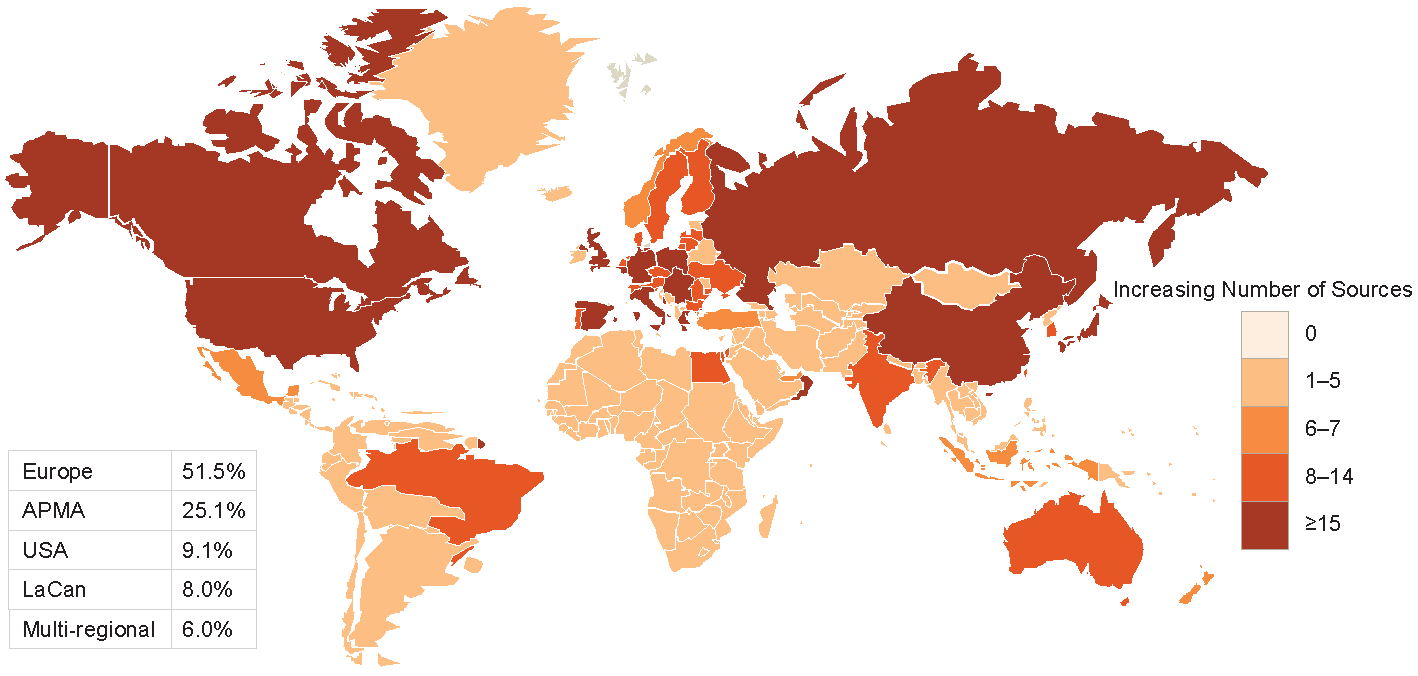
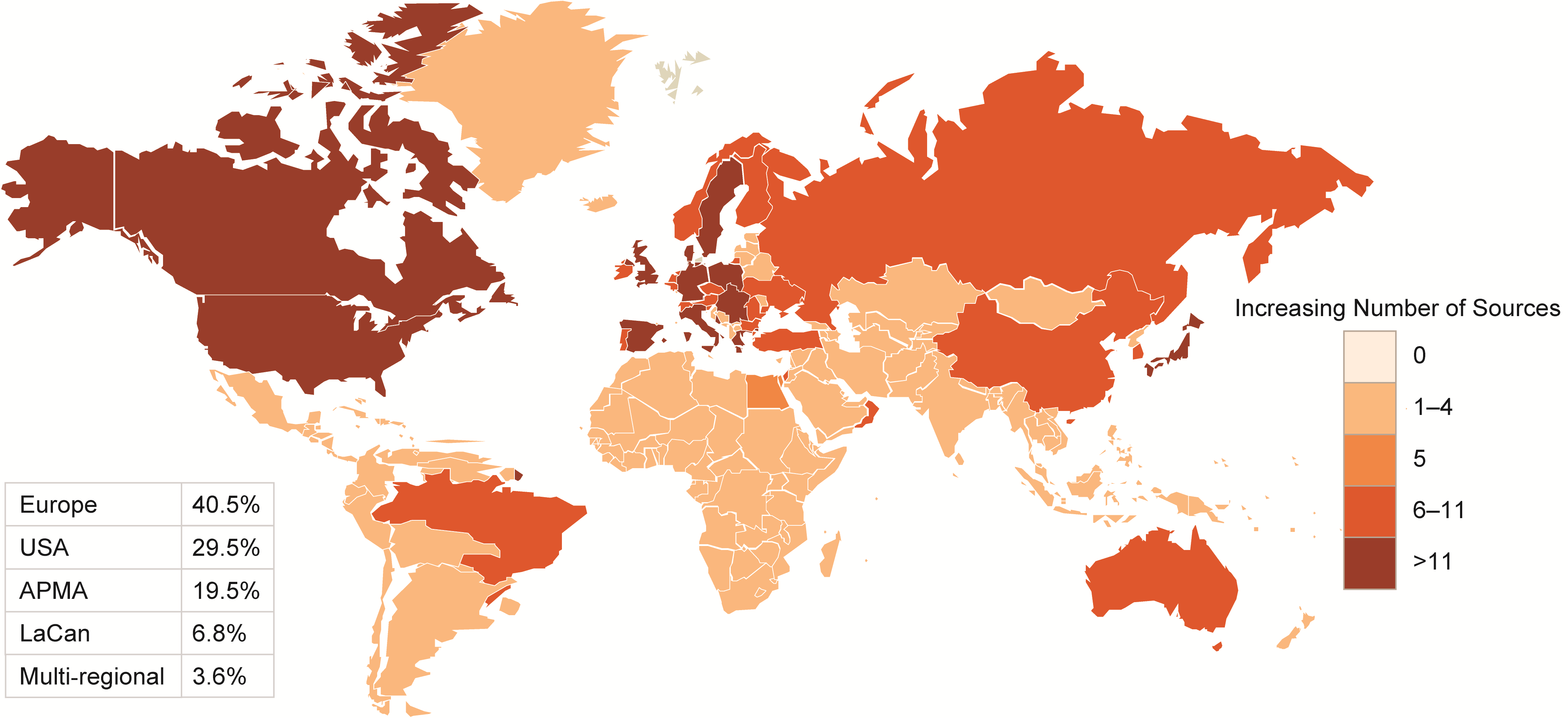
A detailed review was conducted of 322 (HF), 287 (ACS) and 220 (AF) data sources. The majority of data sources had near-complete data on demographic variables and considerable data on comorbidities. The least reported data categories were drug codes and caregiver involvement. Only a minority of data sources provided information on access to data for other researchers or whether data could be linked to other data sources to maximize clinical impact.
Results of the review
This review has created a comprehensive resource of cardiovascular data sources, providing new avenues to improve future real-world research and paving the way for better patient outcomes.
The list and metadata for RWD sources are publicly available in the Medical Information Framework catalogue¹ after registration. Please select the BigData@Heart Literature Review.
The results are being made available to the scientific and medical communities as a resource on available RWD for AF, ACS, and HF. The information on the data sweeps is intended to be updated by the data holders or an update to the data sweep moving forward in the future.