Background
Depending on which disorder you are looking to identify, Holter monitoring, exercise testing and cross-sectional imaging can be invaluable in reaching a diagnosis.
I - Conduction disorders
1) Bundle branch block (BBB)
Partial right BBB (RBBB pattern but QRS<120ms) is commonly found in trained athletes but care is needed not to miss underlying pathology such as an atrial septal defect, brugada syndrome or arrhythmogenic right ventricular cardiomyopathy (see below) (1). Complete RBBB or LBBB however is uncommon and always requires further investigation, as it can be a marker for underlying disease (Table 1) (2). Resting blood pressure +/- ambulatory monitoring, exercise testing, Holter monitoring and echocardiography are recommended. If no cause is found, a primary degenerative lesion of the conducting system may be the cause and cardiovascular risk factor modification is important. However for LBBB it may be an early manifestation of underlying ischaemic heart disease or cardiomyopathy so patients should also be invited for surveillance echocardiography.
Table 1: Causes of bundle branch block:
- RBBB LBBB
- Hypertension
- Myocardial infarction or ischaemia
- Cardiac surgery
- Congenital cardiac disease
- Myocarditis
- Chronic obstructive pulmonary disease and cor pulmonale
- Pulmonary embolism
- Valvular disease
- Channelopathies
- Cardiac tumours
- Sarcoidosis
- Chagas disease
Bifascicular block or LBBB in young individuals should prompt screening of siblings and consideration of genetic testing to exclude a genetically determined progressive conduction abnormality (e.g. Lènegre disease)(3).
2) Sinus node disease
Sinus node disease encompasses a wide range of clinical presentations from sinus bradycardia to sinus arrest or bradycardia–tachycardia syndrome (4). Most cases occur in patients older than 65 but there are familial cases in which disease occurs at a younger age (5). A clinical history of syncope or presyncope is common although milder presentations with fatigue or dyspnoea, reduced exercise capacity, or cognitive impairment are also possible.
The diagnosis is confirmed by capturing sinoatrial block (pause > 3 seconds) or significant tachy- or brady- arrhythmias on ECG recordings. This may be achieved through carotid sinus massage (risk of neurological complication < 1 in 300 when not performed in those with a history of stroke within the past 3 months, or carotid bruits (6) or more commonly prolonged ECG monitoring (Table 2).
Table 2: European Society of Cardiology recommendations* for prolonged ECG monitoring.
| Type of monitoring | Symptom frequency |
| Holter | > once per week |
| External loop recorder | > once per mont |
| Implantable loop recorder | All others |
* for those presenting with syncope but relevant to many other situations
Exercise ECG is helpful in those presenting with sinus bradycardia (< 40 bpm) to assess for chronotropic response, with the failure to achieve 85% of maximal age-predicted heart rate suggesting sinus node disease.
3) Wolff-Parkinson-White syndrome (WPW)
This can present with palpitations or syncope from an atrioventricular reciprocating tachycardia or rarely sudden death secondary to ventricular fibrillation from rapid conduction of atrial fibrillation across the accessory pathway. However, many cases remain asymptomatic with estimates suggesting that 65% of adolescents and 40% of individuals over 30 years with a WPW pattern on a resting ECG are asymptomatic.
Abrupt loss of pre-excitation (short PR/delta wave) on ECG or exercise ECG indicates a lower risk. Symptomatic patients or those with high-risk features (Table 3) should proceed to electrophysiological studies and ablation but in those not meeting criteria follow-up with counseling on symptom awareness is sufficient . Whilst most have a structurally normal heart it can also be associated with congenital heart disease, rare cardiac tumours or hypertrophic cardiomyopathy and hence echocardiography should be performed (7).
Table 3: High-risk features in WPW syndrome
- Age <30yrs
- Male gender
- History of AF
- History of syncope
- Associated congenital or other heart disease
- Familial WPW (incidentally, this accounts for ~5% of cases)
II - Arrhythmias and channelopathies
1) Atrial fibrillation
AF increases with age and whilst for many there are no clinical sequelae, there is a three-fold increased risk of heart failure, five-fold increased risk of stroke and increased mortality. As a result, opportunistic screening is now recommended for those age 65 years or older with pulse palpation and, if irregular, 12-lead ECG for confirmation (8). In paroxysmal cases Holter monitoring or event recorders may be needed to capture the arrhythmia (9).
Echocardiography is useful in screening for suspected AF as it can identify possible structural causes for arrhythmia such as valvular disease (typically mitral valve), atrial septal defect, cardiomyopathy or an enlarged left atrium from another cause. In confirmed cases of AF echocardiography is recommended. Genetic testing is not recommended for atrial fibrillation (AF), as no specific gene has been found to account for ≥ 5% of disease.
2) Atrial flutter
Typical atrial flutter is caused by a right atrial macro re-entrant circular tachycardia. It shares the same risk for thromboembolic disease as AF and investigation should be identical to that for AF. The ECG criteria for diagnosis are outlined below (Table 4).
Table 4: Atrial Flutter: Typical form (10)
- Negatively directed saw-tooth atrial deflections (f waves) in leads II, III, and aVF
- Positive deflections in V1 in the typical form (counterclockwise atrial flutter)
- Atrial rates of 250 to 350 bpm
- Typically there is 2:1 AV block so the characteristic ventricular rate is 150 bpm
- Variable block may occur leading to an irregular rate.
- Reverse typical form (clockwise isthmus-dependent flutter)
- Positive flutter waves in leads II, III, aVF
- Negative flutter waves in lead V1
- Atypical flutter
- Continuous undulation of the atrial complex
- Not meeting criteria for typical or reverse typical flutter
- Atrial rates >240 bpm
3) Supraventricular tachycardia (SVT)
SVT includes a myriad of different clinical conditions and the most common mechanism for development is re-entry. A description of palpitation is extremely useful and can differentiate between various causes (Table 5).
Table 5: Descriptions of palpitation and possible diagnoses (11)
| Descriptions of palpitation | Possible diagnoses |
| Missed beats or pauses followed by a strong beat | Ectopics |
| Regular rapid beats with abrupt onset and termination | SVT |
| Irregular beats | AF/Flutter/Multifocal atrial tachycardia |
| Regular beats with gradual onset, acceleration and deceleration | Sinus Tachycardia |
A history of termination with vagal manoeuvres is also highly suggestive of a re-entrant tachycardia. The resting ECG should be evaluated for abnormal rhythm, pre-excitation, prolonged QT interval, sinus tachycardia, ST segment abnormalities, or evidence of underlying heart disease. If pre-excitation is present the condition is managed as WPW. If the ECG is normal then consider proceeding to prolonged ECG monitoring (Table 2), or request patients to present for an ECG when symptomatic. Echocardiography should be performed in all cases of documented arrhythmia to exclude an underlying structural cause.
4) Brugada syndrome
Brugada ECGs have been characterised into three patterns (Figure 1). A type 1 Brugada ECG has coved ST elevation of > 0.2mm followed by a negative T-wave (Figure 1A). A downsloping ST segment from an elevated J point with an STJ/ST80 (80ms after J point) ratio >1 is found in the Type 1 ECG pattern. The type 2 ECG has a saddleback morphology with high take-off ST elevation followed by a biphasic (Figure 1B) or positive (Figure 1C) T-wave. A type 3 ECG has either a coved or saddleback morphology with J point elevation >2mm but the terminal portion of ST segment <1mm. Type 2 and Type 3 patterns can be confused with partial RBBB but measuring the angle between the upslope of the S wave and downslope of the r’ wave (β in Figure 2) can be helpful with an angle >58° having a sensitivity of 92% and specificity of 87% in those without structural heart disease (12). Pharmacological testing with a class 1C antiarrhythmic such as Ajmaline can unmask the typical ECG, however it is non-diagnostic in up to 10% of SCN5A mutation carriers. In confirmed cases cascade ECG screening of family members is required and genetic testing can be useful for those with a Type 1 ECG but it is not recommended for those with an isolated type 2 or 3 pattern.
Figure 1: Patterns of Brugada ECG (8) Reproduced by permission of Oxford University Press
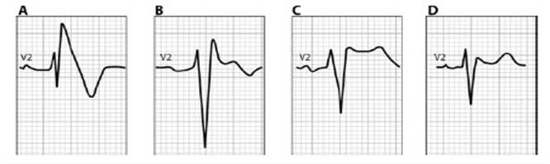
Figure 2: Differentiating Brugada Type 2 or 3 from partial RBBB (19)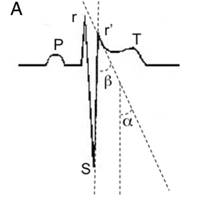
5) Long QT Syndrome (LQTS)
The QT interval should be measured in lead II, V3 or V5. It increases with age and female sex and decreases with increasing heart rate in a non-linear relationship. The corrected (QTc) using Bazetts formula QT/√R-R is therefore inaccurate at high and low heart rates.
A line along the maximum slope of the T wave and the intersection with the baseline marks the end of the QT interval (Figure 3). A QTc > 500ms if otherwise unexplained is diagnostic whereas a QTc > 440ms in men, > 460ms in women raises the possibility of LQTS. Possible causes for a long QT interval (Table 6) should be excluded and an echocardiogram performed to discount a structural cause for delayed repolarisation.
Figure 3: Measurement of the end of the QT interval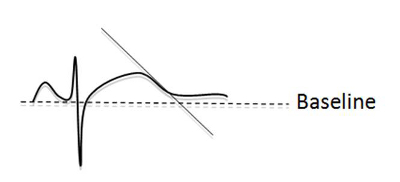
Table 6: Causes of a prolonged QT interval
- Drugs (antipsychotics, Class 1A, 1C and III antiarrhythmics, antidepressants, antihistamines, macrolide antibiotics, quinine and hydroxychloroquine)
- Metabolic disorders (giving rise to alkalosis)
- Electrolyte disturbances (hypo-kaleamia, calcaemia, magnesemia)
Holter monitoring, postural ECG’s and exercise testing can be used in those with a non-diagnostic ECG (13)(14). An increase in QTc on standing of >30msecs has a 67% sensitivity and specificity, whilst end of recovery (4 mins post exercise) QTc >445ms has a sensitivity of 92% and specificity of 88% for identifying LQTS individuals. Once LQTS is confirmed cascade ECG screening of family members should ensue along with genetic testing.
III - Structural disorders
1) Coronary artery anomalies
Coronary artery anomalies are abnormal anatomical findings that overall can be found in 1-2% of a general unselected population (15). Anomalous origination of the artery from the opposite coronary sinus appears to carry the highest risk of sudden cardiac death, particularly in athletic individuals. This can be an anomalous left coronary artery (originating in the right coronary sinus) or anomalous right coronary artery (originating in the left coronary sinus). Whilst the latter is more common (0.92%), the former (0.15%) appears to carry a poorer prognosis. Other high-risk features include an intramural course (typically, but not exclusively between the aorta and pulmonary artery) acute-angled take-off, significant stenosis, or ostial narrowing (16). The majority of cases will be asymptomatic with sudden death the first presentation however some are preceded by exertional symptoms. ECG, Holter and exercise testing are often normal but may show signs of ischaemia or arrhythmia. Echocardiography is more reliable in children than adults but MRI or CT coronary angiography remain the gold standard investigations.
2) Hypertrophic cardiomyopathy (HCM)
Hypertrophic cardiomyopathy is thought to represent 30% of all causes of sudden cardiac death in young athletes (17). It is defined as left ventricular hypertrophy that is typically asymmetric in distribution, showing virtually any diffuse or segmental pattern of left ventricular wall thickening (18). This is associated with a non-dilated and hyperdynamic chamber in the absence of another cardiac or systemic disease, e.g., hypertension, valvular disease, congenital heart disease capable of producing the magnitude of hypertrophy evident.
The ECG can detect at least 90% of cases of HCM (19) demonstrating voltage criteria for left ventricular hypertrophy (LVH), left axis deviation, left atrial hypertrophy, ST segment depression, T wave flattening or inversion in two or more leads, or pathological Q waves. Echocardiography remains the gold-standard investigation, reserving MRI for difficult cases. Echocardiographic criteria include LV wall thickness > 15mm with a normal cavity size. A wall thickness of 13-15mm can be seen in the athletic heart but can also be an early sign of progression to HCM and requires observation and serial echocardiography (20). Patients confirmed to have HCM should be further assessed with Holter monitoring and an exercise test to aid in risk stratification (Table 7). Furthermore, genetic analysis should follow, and can aid in family screening due to autosomal dominant transmission (21).
Table 7: Risk Factors for Sudden Death in HCM (18)
| Major risk factors | Possible in Individual patients |
| Cardiac arrest (ventricular fibrillation) | Atrial fibrillation |
| Sustained ventricular tachycardia | Myocardial ischemia |
| Family history of premature sudden death | High-risk mutation |
| Unexplained syncope | LV outflow obstruction |
| LV thickness ≥30 mm | Intense (competitive) physical exertion |
| Abnormal exercise blood pressure | |
| Nonsustained ventricular tachycardia (Holter) |
3) Dilated cardiomyopathy (DCM)
DCM is defined as the presence of left ventricular dilatation and left ventricular systolic dysfunction in the absence of abnormal loading conditions (hypertension, valve disease) or coronary artery disease sufficient to cause global systolic impairment. In addition to genetic causes, acquired causes such as myocarditis, Kawasaki disease, Churg Strauss syndrome, viral persistence, drugs (eg.anthracyclines), pregnancy, endocrine, nutritional deficiency, alcohol and tachycardiomyopathy (22), should be considered and excluded.
Voltage criteria for LVH is found in 69% of cases but more specifically a ratio ≥ 3 of the R-wave in V6 to maximum R wave in lead I, II or III is found in 67% of cases. This ratio is found in only 4% of patients with valvular heart disease and 1% of those with hypertension related LVH (23).
Echocardiography should establish the ejection fraction <45%, and/or a fractional shortening <25%, in association with left ventricular end-diastolic dimension > 112% predicted when corrected for age and body surface area. Holter monitoring and exercise testing to look for arrhythmias and assess exercise capacity is recommended (24). Genetic testing is advised for those with cardiac conduction disease or a family history of premature unexpected sudden death and can be useful in familial cases to determine those at highest risk of arrhythmia and help with family planning.
4) Arrhythmogenic right ventricular cardiomyopathy (ARVC)
There have recently been modifications to the task force criteria for diagnosis of ARVC. The ECG is abnormal in >90% of cases, and there are now specific ECG, echocardiographic and MRI criteria for diagnosis (25). Investigations should include a detailed family history, ECG and signal averaged ECG (SAECG), echocardiography with cardiac MRI if technically inadequate or inconclusive, Holter monitoring and endomyocardial biopsy if needed. Key findings include inverted T waves and epsilon waves (reproducible low-amplitude signals between end of QRS complex to onset of the T wave) in the right precordial leads (V1-V3), regional RV akinesia, dyskinesia or aneurysm and non-sustained or sustained ventricular tachycardia of left bundle-branch morphology on Holter monitoring. Genetic testing can be useful for those with a confirmed diagnosis and may be indicated for those with a possible diagnosis.
IV - Other conditions
Coronary Heart Disease
Atherosclerotic cardiovascular disease, of which coronary heart disease is a substantial proportion, is now the leading cause of death worldwide (26). It is a recognised cause of sudden death in young athletes and is not usually detected by simple screening methods. Cardiovascular risk assessment based on standard risk factors (hypertension, diabetes, smoking, cholesterol, age, sex) should be taken into account and the patient can be risk stratified according to standard tables. Exercise testing may help in risk stratification. Parameters such as T-wave alternans (beat-to-beat alternation in the shape, amplitude, or timing of the ST segment and the T wave) ≥ 60μV during exercise, heart rate recovery ≤18 bpm during the first 60 secs of recovery (27), or ST depression ≥1mm (28) are associated with increased cardiovascular risk.
When screening asymptomatic, low risk individuals, CT calcium scoring has a high negative predictive value. If the score is zero it is nearly 100% certain that there is no significant coronary artery narrowing. However, the presence of coronary calcium has a specificity of only 50% to detect >50% coronary artery stenosis so subsequent CT coronary angiography can be used to determine high or low risk disease in those with an elevated score. Overall, CT may therefore enhance classification of risk (29), which can be used to guide aggressive risk factor modification and potentially intervention in those with prognostically significant disease.
Conclusions
When screening for specific inherited disorders we need to tailor our history, examination and investigations accordingly. ECG and prolonged ECG monitoring can detect conduction disorders, arrhythmias and channelopathies although we need to be mindful of occult ECG changes that require further evaluation. The ECG can also detect cardiomyopathies and ECG changes may precede structural changes seen with echocardiography so further surveillance is necessary in these cases. There are a number of diseases however in which echocardiography is still the gold-standard diagnostic test and perhaps we will see a greater use of hand-held echo as a screening tool in the future. Coronary artery anomalies are notoriously difficult to screen for but where there is clinical suspicion, CT or MRI scanning should be performed. Finally, detecting coronary artery disease in asymptomatic individuals is challenging but with the emerging use of CT calcium scoring and CT coronary angiography, risk stratification may be enhanced to potentially enable primary intervention in those with prognostically significant disease.


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.