The heart is the first organ that displays morphological asymmetry during embryonic development. However, molecular asymmetry is initiated far before any morphological differences can be observed. Such initial laterality signals will directly contribute to the asymmetric morphogenesis of distinct mesodermal derivates, including the developing heart. Over the last years, extensive insight has been gained concerning the early cellular and molecular cues that drive the establishment of laterality. However it remains rather elusive how such initial laterality cues are projected into the developing heart and how much they contribute to cardiac morphogenesis.
In this short essay, we will review current data regarding early processes of left-right patterning and their contribution to cardiac precursor cell deployment, chamber formation and cardiac conduction system development during formation of the embryonic heart.
Early laterality signals
A directional flow of a yet undetermined factor, driven by the ciliated cells of the node [Hamada et al., 2002; Nonaka et al., 2005], initiates the activation of nodal expression in left lateral plate mesoderm [Shiratori et al., 2001; Saijoh et al., 2003]. This, in turn, activates lefty-2 expression [Meno et al., 1998; 1999] and leads to the transcriptional activation of the homeobox transcription factor Pitx2 within the lateral plate mesoderm [Yoshioka et al., 1998]
Figure 1:
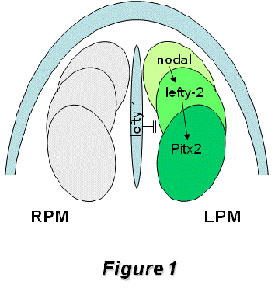
Figure1: Schematic representation of the left-right signaling pathway operating during early left-right symmetry breaking in the developing mouse embryo. Nodal expression is initiated on the left side of the node and amplified within the left lateral plate mesoderm, which in turn activates lefty-2 and soon thereafter the homebox transcription factor Pitx2. Midline expression of lefty-1 inhibits nodal and lefty-2 expression transfer to right lateral plate mesoderm. While nodal, lefty-2 and lefty-1 expression are extinguished before organogenesis is initiated, Pitx2 remains expressed at these stages, including organ primordia such as the heart, gut and the lungs
This nodal/lefty/Pitx2 signaling cascade is highly conserved across evolution [Boorman and Shimeld, 2002a, 2002b]. Genetic manipulation of a myriad of genes within this early left-right pathway has provided evidence of the complex regulatory networks involved in establishing laterality [see Hamada et al., 2002 for a review]. Interestingly, loss of many of these genes has a clear impact on cardiogenesis provoking right or left atrial isomerism. However, it remains to be established if other cardiovascular defects such as double-outlet right ventricle (DORV) and transposition of the great arteries (TGA), which are also observed with relatively high frequency in mouse laterality mutants, are directly caused by left-right signaling impairment [Franco and Campione, 2003] and if so, which molecular cascades are involved.
This nodal/lefty/Pitx2 signaling cascade is highly conserved across evolution [Boorman and Shimeld, 2002a, 2002b]. Genetic manipulation of a myriad of genes within this early left-right pathway has provided evidence of the complex regulatory networks involved in establishing laterality [see Hamada et al., 2002 for a review]. Interestingly, loss of many of these genes has a clear impact on cardiogenesis provoking right or left atrial isomerism. However, it remains to be established if other cardiovascular defects such as double-outlet right ventricle (DORV) and transposition of the great arteries (TGA), which are also observed with relatively high frequency in mouse laterality mutants, are directly caused by left-right signaling impairment [Franco and Campione, 2003] and if so, which molecular cascades are involved.
Cardiac morphogenesis and laterality genes
The development of the heart is a complex process, initiating soon after gastrulation as precardiac progenitor cells arise symmetrically within the cardiac crescent of the developing embryo [Garcia-Martinez and Schoenwolf, 1993]. Soon afterwards, these precardiac cells migrate towards the embryonic midline adopting a linear heart tube configuration. At this stage, the heart displays the earliest morphological sign of asymmetry apparent in the embryo, as the linear tube bends to the right, configuring thereafter distinct atrial and ventricular cardiac chambers [Christoffels et al., 2001]. The separation of each embryonic chamber as well as their connecting counterparts results in the formation of a four-chambered heart with distinct systemic and pulmonary circuitries [Franco et al., 1998]. The homeobox transcription factor Pitx2 is the only component of the early left-right signaling cascade that continues to be expressed in the cardiac crescent and early heart tube [Piedra et al., 1998; Logan et al., 1998; Yoshioka et al., 1998; Campione et al., 1999]. Thus, understanding the role of Pitx2 during cardiac morphogenesis will contribute to understanding the impact of laterality during cardiac development. Further insights regarding the early left-right determinants during gastrulation can be found in excellent recent reviews [Hamada et al., 2002; Shiratori and Hamada, 2006].
Pitx2 and cardiac precursor cells
At the time that the first cardiac precursor cells are located symmetrically at each side of the developing embryo, there is already a distinct left-right asymmetric expression of Pitx2. Soon after, the cardiac precursor cells will migrate ventrally towards the midline to form an antero-posterior polarized linear heart tube. Pitx2 expression remains confined to the left side of the linear straight tube, supporting a directly contribution of the left cardiac crescent to the left side of the cardiac tube, as demonstrated by direct cell tracing experiments in the chick [Campione et al., 2001]. Interestingly, lack of Pitx2 at these early stages does not significantly alter cardiac precursor cell commitment and/or proliferation [Lin et al., 1999; Lu et al., 1999].
Heart development is a dynamic process and thus it requires the progressive deployment of distinct progenitor cell pools, referred to as cardiac fields. It has been recently demonstrated that all cardiac embryonic cells are derived from early Nkx2.5/Isl1 positive progenitor cells [Zhou et al., 2008; Ma et al., 2008]. However, the genetic contribution of these transcription factors seems to be distinct in different precursor cell populations. Accordingly, the early cardiac crescent, i.e. the first heart field, is mainly characterized by robust Nkx2.5 expression while Isl1 expression seems to be dispensable for initial heart tube formation [Cai et al., 2003]. The early cardiac crescent mainly contributes to the left (systemic) ventricle in the embryonic heart [Meilhac et al., 2003]. A second pool of cells, termed the second heart field, is characterized by robust Nkx2.5 and Isl1 co-expression [Cai et al., 2003], and contributes to the right ventricle/outflow tract and atrial chambers [Kelly et al. 2001; Meilhac et al., 2004; Buckingham et al., 2005]. Recently, a third population of cells, characterized by robust Tbx18 expression but neglible Nkx2.5/Isl1 expression, has been reported to contribute to the caval vein myocardium, most posterior part of the heart, suggesting the existence of a third heart progenitor cell field [Christoffels et al., 2006]. Interestingly, Pitx2 is asymmetrically expressed in all these cardiac progenitor cell populations [Campione et al., 2001]
Figure 2:
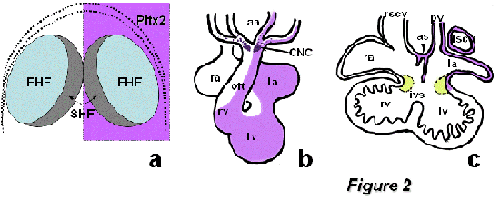
Figure2: Schematic representation of the homeobox transcription factor Pitx2 during cardiogenesis. Panel A represents Pitx2 early expression, which is broadly observed within both the first (FHF) and second (SHF) heart field precursors. Panel B illustrates the asymmetric remodeling of Pitx2 expression in the embryonic looped heart, while panel C displays the remnants of Pitx2 expression in atrial structures at fetal stages. aa, aortic arches; CNC, cardiac neural crest; ias, interatrial sepum; ivs, interventricular septum; la, left atrium; lv, left ventricle; lscv, left superior caval vein; oft, outflow tract; pv, pulmonary vein; ra, right atrium; rscv, right superior caval vein; rv, right ventricle.
Functional characterization of the role of Pitx2 during cardiovascular development has been extensively explored by gene inactivation in mice [Lin et al., 1999; Lu et al., 1999] (see Table I). Systemic Pitx2 null mutants display an overt embryonic phenotype, characterized by a wide array of embryonic defects, including cardiovascular defects such as right atrial isomerism, DORV and TGA [Lin et al., 1999; Lu et al., 1999]. Isoform-specific Pitx2 deletion revealed that Pitx2c is the most relevant isoform for correct cardiac development, whereas Pitx2a and Pitx2b seemed to be dispensable [Liu et al., 2001, 2002]. Conditional deletion of Pitx2 in distinct tissue layers has provided evidence that Pitx2 is required within cardiac precursor cells [Ai et al., 2006] as well as within cardiac myocytes themselves [Tessari et al., 2008] but not in the migrating cardiac neural crest cells [Ai et al., 2006], as had been previously suggested [Kioussi et al., 2001]. Importantly, while lack of Pitx2 in early cardiac precursor cells largely recapitulates the early embryonic systemic phenotype, Pitx2 loss of function in developing cardiomyocytes asymmetrically impairs myofibrillar architecture from relatively early developmental stages, but does not lead to severe morphogenetic alignment defects, such as DORV or TGA [Tessari et al., 2008].
Chamber formation and Pitx2
The role of Pitx2 during chamber formation was initially explored on the basis of its expression profile during normal and abnormal cardiac development. Campione et al. [2001] demonstrate that the expression of Pitx2 dynamically changes during development as the heart is remodeled from a symmetrical tube into a chambered heart. Interestingly, Pitx2 expression at early heart tube stages is confined to the left cardiac portion while as the heart loops to the right, left-right differential expression is maintained as such in the developing atrial chambers, but is converted into dorso-ventral differences in the developing ventricular chambers. Furthermore, ectopic (symmetric) atrial and/or ventricular Pitx2 expression was significantly associated with the presence of congenital heart defects such as DORV and TGA in the iv/iv mouse laterality mutant model, suggesting that these cardiac abnormalities might be linked to impairment of left-right signaling (Campione et al., 2001; Franco and Campione, 2003). These observations are consistent with the cardiac congenital malformations observed in Pitx2 null mutants (Lin et al., 1999; Lu et al., 1999), providing further support for a causative relationship. However, genetic screening of a relatively small cohort of TGA human patients have to date provided no evidence of Pitx2 coding sequence mutations [Muncke et al., 2005], although further experiments are required to screen for putative mutations in non-coding and regulatory sequences that might be relevant for Pitx2 expression. Interestingly, genome-wide screening has identified genetic variants within the vicinity of Pitx2 locus which are significantly associated with patients with atrial fibrillation, opening new avenues for the role of Pitx2 in cardiac physiopathology [Gudbjarston et al., 2007].
Conditional deletion of Pitx2 in the mouse using Cre/LoxP technology has provided further evidence as to the role of Pitx2 during cardiovascular development. Ai et al [2006] have reported that lack of Pitx2 using both Mef2c-Cre and Islet1-Cre deletor mouse strains, provokes similar cardiac defects to those observed in Pitx2abc and Pitx2c null mutants, supporting the notion that Pitx2 plays an important role since its appearance in the cardiac progenitor cells. Furthermore, Tessari et al. [2008] have recently provided evidence that Pitx2 function is also required within differentiated cardiomyocytes, as revealed using a MHC-Cre deletor mouse strain. Tessari et al. [2008] nicely demonstrated that Pitx2 is required in the left atrial chamber to repress Bmp10 expression while in the ventricular chamber Pitx2 plays a determinant role on the myofibrillar architecture of Pitx2 positive ventricular myocytes.
Further evidence of chamber-specific roles of Pitx2 during cardiac development have come from gain-of-function experiments performed in second heart field explants as reported by Galli et al. [2008]. These authors demonstrate that Pitx2 plays an essential role in controlling asymmetric expression of an Mlc3f-lacZ transgene in left but not right atrial precursor cells and furthermore that Pitx2 regulates cell cycle proliferation within left but not right precursor cells. Thus, these data reinforce the notion that Pitx2 plays critical roles in controlling left-right asymmetric expression of cardiac enriched genes as well as in the morphogenetic processes underlying left-right atrial chamber formation and identity.
Mommersteeg et al. [2007] have revealed that Pitx2 plays a critical role in the morphgogenesis of the venous pole of the heart consistent with previous reports describing asymmetric expression of this transcription factor in the inflow tract of the heart [Franco et al., 2001]. These authors have provided evidence that Pitx2 is required for the initial proliferation and subsequent expansion of the pulmonary vein myocardial precursors, as Pitx2 deficient mouse embryos fail to properly develop the myocardial sleeve surrounding the pulmonary veins. Furthermore these authors demonstrated that coordinated action of Nkx2.5 and Pitx2c is required for formation of the pulmonary but not systemic venous return [Mommersteeg et al., 2007].
Cardiac conduction system, Pitx2 and laterality
Novel insights regarding the impact of embryonic laterality on the formation of the cardiac conduction system have been recently reported, even though this part of the heart has not previously been referred as an left-right determined structure. Mommersteeg et al. [2008] have recently demonstrated that Pitx2c plays a critical role in the establishment of the sinoatrial node gene expression programme in the right but not left atrial sinoatrial junction. In the absence of Pitx2, bilateral right atrial chambers are formed, including therein bilateral sinoatrial nodes. In this context, Mommersteeg et al [2008] nicely demonstrate that Nkx2.5 expression is required to limitTbx3 expression in the forming sinoatrial node, and that Pitx2 left-right asymmetric expression superimposes a left-right differential programme, by repressing a default right atrial chamber-SAN forming programme in Pitx2-expressing left atrial myocardium. Pitx2 is therefore part of the mechanism restricting pacemaker activity in the developing heart and SNPs have recently been reported proximal to the human PITX2 locus that has been associated with atrial fibrillation. Interestingly, it remains to be established if Pitx2 lateralization effect might also contribute to the formation of other components of the cardiac conduction system such as the atrioventricular node and the left and right atrioventricular bundle branches
Figure 3:
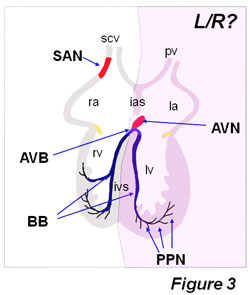
Figure3: Distribution of the adult cardiac conduction system components. Note that left-right (L/R) signal asymmetrically can influence the development of the sinoatrial node (SAN), as it has been nicely demonstrated by Mommersteeg et al (2008). However, it remains to be explored if left-right signaling can also affect other cardiac conduction system components, such as the atrioventricular node (AVN), atrioventricular bundle (AVB) and left and right bundle branches. Based on our studies using left/right ventricular markers [Franco et al., 2006], we would propose that the AVN and proximal part of the AVB and the left bundle branch might be derived from the first heart field and thus less exposed to asymmetrical signals, whereas the distal part of the AVB as well as the right bundle branch may be derived from the second heart field and thus likely to be prone to be impaired by laterality defects. BB, bundle branches; ias, interatrial sepum; ivs, interventricular septum; la, left atrium; lv, left ventricle; PPN, peripheral Purkinje fiber network; scv, superior caval vein; pv, pulmonary vein; ra, right atrium; rv, right ventricle.
Perspectives
Understanding the genetic contribution of Pitx2 during cardiac development has provided a new framework to unravel the impact of embryonic left-right signaling within the developing heart. Isoform-specific Pitx2 mutants have demonstrated that Pitx2c is the most important isoform during cardiac development. The generation of spatio-temporal conditional Pitx2 mutants has demonstrated that Pitx2 is not required in cardiac neural crest cells, whereas Pitx2 is crucial at both precardiac and myocardial stages. Of note, the impact of Pitx2 deletion is more severe if the second heart field is affected as compared to the first heart field. Furthermore, it remains to be established if the role of Pitx2 is distinct in discrete myocardial compartments. It is highly surprising to notice that atrial isomerism and abnormal venous return is observed only if cardiac precursor cells are depleted of Pitx2, whereas depletion in the myocardium does not alter the morphology of the atrial chambers or the venous return. Further fine tuned dissection of the temporal aspect of Pitx2 function is required to pursue these observations. Furthermore, conditional deletion of Pitx2 in endocardial, atrial and ventricular myocardial compartments will provide further insights into the role of Pitx2 during cardiac development
Figure 4:
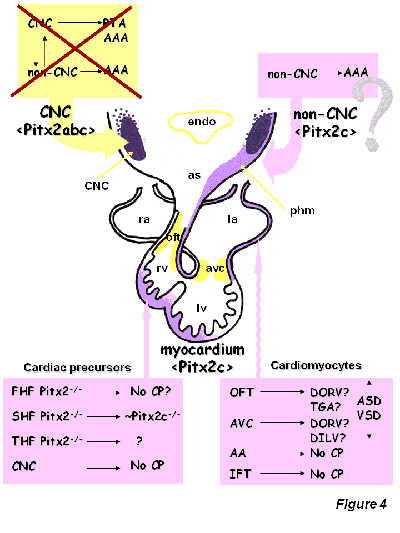
Figure4: Schematic drawing of the putative role of Pitx2 during cardiac morphogenesis (modified after Franco and Campione, 2003). FHF, first heart field, SHF, second heart field; THF, third heart field; CNC, cardiac neural crest; myo, myocardial component. No CP, no cardiac phenotype. OFT, outflow tract, AVC, atrioventricular canal, AA, aortic arches, IFT, inflow tract; DORV, double outlet right ventricle; DILV, double inlet left ventricle; TGA, transposition of the great arteries; ASD, atrial septal defects; VSD, ventricular septal defects, phm, pharyngeal mesoderm
Surprisingly, to date, there is a bulk of information regarding the morphogenetic defects caused by Pitx2 loss-of-function, while there is little information about the cellular and molecular mechanisms controlled by this homeobox transcription factor during cardiac development. Only one gene, Nppa, encoding for the atrial natriuretic factor (ANF), has been demonstrated to be a direct Pitx2 target using an in vitro approach, whereas indirect evidence for Bmp10 deregulation in vivo suggests that this gene may also be a Pitx2 target. Thus, further investigation is called for into the cellular and molecular processes regulated by Pitx2.

 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.