Current therapeutic strategies in patients with ST-segment elevation myocardial infarction (STEMI) aim for timely recanalisation of the infarct-related artery to reduce the progression of the ischemic-necrotic wavefront of myocardial injury (1,2).
However, reperfusion after prolonged occlusion does not always translate into adequate vascularisation of the microvascular bed. This phenomenon is called micro-vascular obstruction (MVO) or no-reflow and has been shown both in experimental and clinical studies (3,4). Previous studies have shown that no-reflow is an independent predictor of left ventricular remodelling and poor prognosis (5,6) therefore accurate assessment is vital.
There are several techniques to assess no reflow including:
- Qualitative assessment of coronary angiography by means of antegrade angiographic epicardial blood flow graded by TIMI flow (7), corrected TIMI frame count (CTFC) and myocardial blush grade (MBG) (8).
- Myocardial contrast echocardiography: a persistent contrast defect despite a patent epicardial artery after reperfusion correlates with the MVO (9).
- On the other hand, cardiovascular magnetic resonance (CMR) with standard gadolinium chelates administration has proved useful for detection of the occurrence of the no-reflow phenomenon in patients with revascularised STEMI.
Indeed, the use of magnetic resonance imaging in the management of acute myocardial infarction has changed the therapeutic approach to patients affected by STEMI. When patients present outside the diagnostic window of cardiac troponins, delay enhancement- cardiovascular magnetic resonance (DE-CMR) may be especially useful. Moreover, because DE-CMR can uniquely differentiate between ischemic and various non-ischemic forms of myocardial injury, it may be helpful in cases of diagnostic uncertainty, such as in patients with classical features of myocardial infarction (MI) in whom coronary angiography does not show a culprit lesion. Even after the diagnosis of MI has been made, CMR provides clinically relevant information by identifying residual viability, microvascular damage, stunning, and right ventricular infarction (10). In addition, the high spatial resolution of DE-CMR even enables visualisation of microinfarctions, which may occur during otherwise successful percutaneous coronary interventions (11).
1 - Mechanisms of the no-reflow phenomenon
The patho-physiologic mechanism of the no-reflow phenomenon is likely multi-factorial. It derives from a balance between benefits and damage from myocardial reperfusion, depending on the timing of the occlusion. If reperfusion occurs beyond 12 hours, the damage created by free radicals and the release of calcium ions exceeds the benefits of reperfusion. In particular, prolonged ischemia results in endothelial damage, with endothelial cell disruption, lure of neutrophils, red blood cells and platelets that cause microvascular obstruction. Microvessel rupture causes extravasation of these cells and further compression of other capillary. These changes lead to reduced perfusion with death of surrounding viable myocytes (12). Another important factor is the damage caused by the distal coronary microembolisation of plaque and thrombus debris following angioplasty. The rate of coronary microembolisation is highest in documented epicardial coronary thrombosis, reaching 30% to 54% (13,14) and even a higher rate (79%) in STEMI patients (15). Angiographic evidence of distal embolisation has been shown to occur in approximately 15% to 19% of patients treated with primary percutaneous coronary intervention (PCI) (16,17).
Micro-vascular obstruction is not a stable phenomenon but varies over time and space. It first develops in the core of the infarcted region and then extends towards the epicardium. In addition, it progresses over time and a further increase in the size of a MVO has been demonstrated up to 48 hours after initiation of reperfusion (18).
2 - Assessment by cardiovascular magnetic resonance
Techniques used to detect a MVO in cardiovascular MRI are both based on gadolinium contrast enhancement. The first uses first-pass perfusion of gadolinium: there is an uniform increase of signal intensity in the normal and infarcted area, while there is an area of hypointensity at the core of the infarction (early MVO) (Figure 1). The second technique concerns the use of late enhancement. After a wash-out period of approximately 10-12 minutes from intravenous bolus of contrast, the infarcted area is highlighted as an area of hyperenhancement or "bright" area, while the MVO appears as a central area of hypointensity within the infarct hyperintensity (Figure 2-3). The technique of first-pass has proved more sensitive than late-enhancement, because at times, the gadolinium can not penetrate into the zone of the MVO and consequently will underestimate the affected area (19).
3 - Prognostic impact of no-reflow
The presence of no-reflow in patients with acute myocardial infarction has been found to be a predictor of adverse events, with higher incidence of left ventricular (LV) remodelling, congestive heart failure, and death. An initial study by Wu et al. (n. 44; follow-up 16 months) demonstrated that patients with MVO had more cardiovascular events (45% versus 9%, P 0.016) independently of the total infarct size (6). Since then, several studies have succeeded in demonstrating such a correlation. A larger study by Hombach et al. (20) found that infarct size, MVO, LV end-diastolic volume, and EF predicted major adverse cardiac events (MACE), with MVO being the strongest predictor (13.2% more events). Cochet et al (21) showed that MVO and the Global Registry of Acute Coronary Events (GRACE) score were significant predictors of MACE (odds ratio [OR], 8.7; CI, 3.6 to 21.1; P<0.001; OR, 2.8; CI, 1.3 to 6; P<0.01, respectively). Nijveldt et al. (22) examined the relation between angiographic, electrocardiographic, and gadolinium-enhanced CMR characteristics of MVO and they found that early and late MVO were both related to incomplete ST-segment resolution (6 (17%) versus 30 (83%), p < 0.002 and 11 (31%) versus 25 (69%), p < 0.01, respectively) but not to TIMI flow grade and MBG. Among angiographic, electrocardiographic and CMR variables, late MVO was the strongest parameter to predict changes in LV end-diastolic volume (β = 0.53; p < 0.001), end-systolic volume (β = 8.67; p < 0.001), and ejection fraction (β = 3.94; p < 0.006) at follow-up.
Another fundamental point is the role of primary PCI. Francone et al. (23) showed that shorter time-to-reperfusion was associated with smaller infarct size and microvascular obstruction and larger salvaged myocardium. In particular MVOs were larger in patients reperfused later (0.5%, 1.5%, 3.7%, and 6.6%, in < 90, min, > 90 to150 min > 150 to 360 min and > 360 min respectively, p < 0.047), suggesting that any strategy to shorten the delay in the reperfusion of patients with STEMI is crucial.
Figure 1. Short-axis gradient echo images
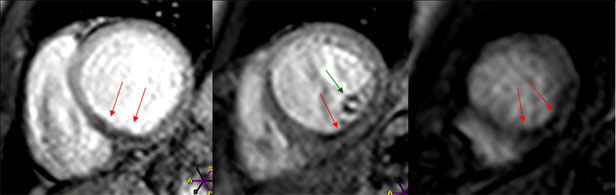
Short-axis gradient echo images at base, mid and apical ventricular level during rest first-pass perfusion scan, showing a localised microvascular obstruction as a dark rim (red arrows) in the posteroseptal and inferior LV wall (early MVO).
A concomitant involvement of the postero-lateral papillary muscle is also evident (green arrow).
Figure 2. Short-axis late enhancement images
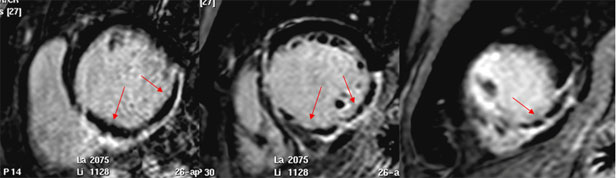
Short-axis late enhancement images at base, mid and apical ventricular level, showing a large area of hyperenhancement (infarct size), with a central zone of hypoenhancement due to microvascular obstruction (late MVO).
Figure 3. Late enhancement images
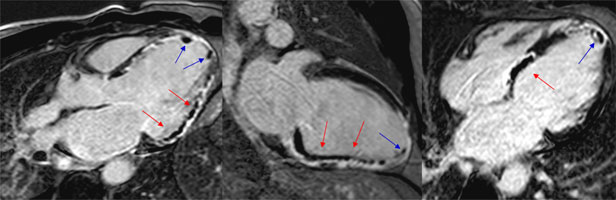
Late enhancement images of LVOT, two chamber and four chamber views, showing a large area of hyperenhancement (infarct size), with a central zone of hypoenhancement due to microvascular obstruction (late MVO, red arrows).
An apical thrombus is evident as a dark mass (blue arrows).
Conclusion:
Contrast-enhanced CMR is a useful non-invasive technique for assessing the presence and extent of microvascular obstruction. No-reflow phenomenon has important prognostic implications, and the use of CMR can help to identify and quantify areas of microvascular damage in patients with STEMI. It could become a powerful tool to stratify the risk of patients and in future to differentiate the effectiveness of different techniques of reperfusion.



 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.