I - From 2D-echo to 3D-echo
2D echo
Left ventricular ejection fraction and volume measurements provide information regarding the prognosis and management of a large proportion of patients suffering from cardiovascular diseases (1-4) and 2D-echo is the most frequently used diagnostic tool (5). However, this imaging technique is limited because it relies on geometric assumptions to calculate the diferent parameters which may introduce important errors.
First-generation scanners for 3D-echo
3D-echo methods have been developed to aquire multiple slices of the left ventricle precisely to avoid having to do geometrical assumptions. But to calculate left ventricular volumes, first-generation scanners offer such limited image quality that offline manual tracings of endocardial borders are still needed when using these scanners. Furthermore, limited computer technology causes data processing to be slow.
Second-generation scanners for 3D-echo
Second-generation full matrix-array transducers have helped to overcome these limitations. These scanners are made up of 3000 simultaneously transmitting elements, that are capable of near real-time acquisition of larger pyramidal 3D datasets from an apical acoustic window. They offer improved image quality and spatial resolution, improved endocardial visualisation with harmonic imaging and larger pyramidal volumes.
II - Data analysis of 3D-echo images
Semiautomated detection of left ventricular volumes and ejection fraction
Until now, data analysis of 3D-echo images had been based on a 2D approach, relying on the selection of 2D-echo images obtained from the apical views and on a manual or semiautomated tracing of endocardial borders. This was a very time-consuming procedure that made it unsuitable for daily clinical practice.
Recently, a new technique for semiautomated detection of left ventricular endocardial borders from 3D images obtained from transthoracic 3D-echo datasets is available. It is now feasible to perform a quantitative assessment of left ventricular volume and ejection fraction with accurate results.
How this technique works
To use this new method, data needs to be acquired in the full volume mode. This is a near real-time technique that requires temporal registration of a number of sub-segments acquired from four consecutive beats.
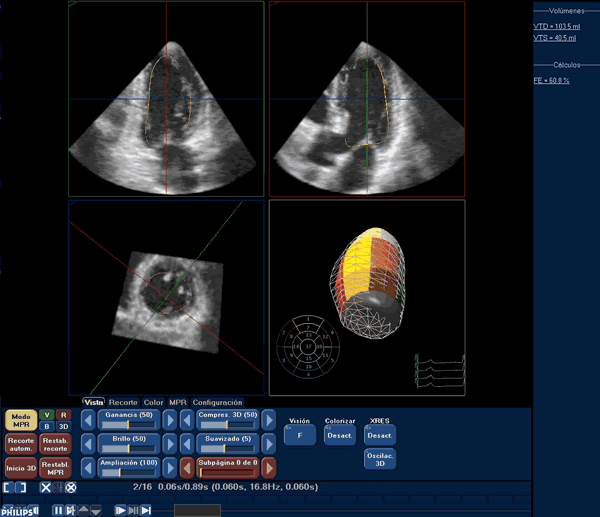
It analyses 3D-echo datasets by performing a semiautomated left ventricular endocardial surface detection. The operator needs only to orientate the major axis of the left ventricle and to mark five points in the endocardial surface: the mitral anterior, inferior, lateral and septal annulus and the apex.
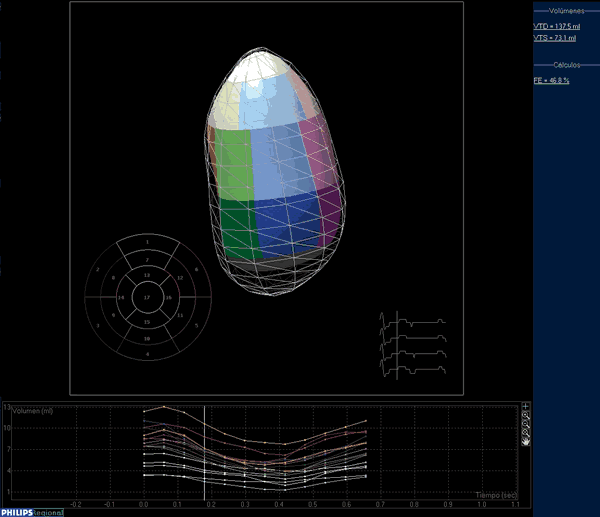
This unique approach allows to speed up the process, which only requires a manual selection of 5 points at different levels in two long-axis planes. Once this selection is completed, the endocardial surface is traced (figure 1) and manual modifications can be made in order to correct any detection defect found by the operator. The resulting left ventricular volumes can be visualised from different points of view, and can be quantified. Furthermore, the global left ventricular volume can be divided into 17 segments so as to be able to assess the alterations in the regional systolic function of the left ventricle (figure 2).
Limitations
However, the fact that this type of data analysis requires temporal registration of a number of sub-segments acquired from four consecutive beats limits this technique.
Patients with severe dyspnoea, atrial fibrillation and cardiac arrhythmias, for instance, cannot easily be studied in this way. And despite the increased width of the pyramidal scan volume available with second generation matrix transducers, patients with severely dilated hearts cannot be adequately assessed either. Further studies need to be performed to better define the applicability of this technique in these populations.
Regarding costs, it is useful to keep in mind as well that to perform 3D-echo, a specific device and probe are necessary, whose costs are superior to conventional ones. Once these tools are acquired, the time necessary for each examination is similar to a conventional echo study.
The content of this article reflects the personal opinion of the author/s and is not necessarily the official position of the European Society of Cardiology.
Conclusion:
Temporal resolution of 3D-echo images is reduced in comparison to 2D-echo images but 3D-echo images render increased spatial resolutions that allow more accurate measurements. The major limitation of our analysis technique remaining is the need to manually initialise the endocardial surface, which is a subjective procedure. Nevertheless, this technique is fast and reproducible.



 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.