In spite of the high reported success rates, catheter ablation for supraventricular arrhythmias remains difficult, with early and late recurrences and complications.
Several strategies to minimise the latter can be followed, such as investigating alternative energy sources, using intracardiac echocardiography to monitor catheter handling and transseptal puncture, or as has became possible only recently, stereotactic navigation of the catheter without manual force.
The restrictions of the present radiofrequency technology are still considerable.
Apart from the fact that lesions are unpredictable and irreversible, catheters need a conductor to bring the energy to the tip.
Additionally, they are equipped with wires to deflect the catheter, sometimes in multiple directions. This explains why with such stiff, still poorly maneuverable catheters perforations of heart and vessels may occur (1).
This results in hematomas, and pericardiac tamponade, not only in unexperienced hands. A further restriction is the lengthy procedure time, associated with high X-ray exposure, which is a potential hazard for the patient, and for the physician.
These considerations are especially important in more difficult situations, as in the presence of anatomic variations (e.g. complex congenital heart disease) or when a difficult target such as atrial fibrillation is approached.
A similar problematic situation can be encountered when attempts are made to implant a biventricular pacing lead in the coronary sinus in a very large heart.
Automation, and remote navigation of flexible soft catheters might be an answer to some of these problems.
This has been made possible with the development of the so-called Niobe system (Stereotaxis Inc, St Louis), which makes it possible to steer a soft catheter with a magnetic tip in the heart, guided by two external, strong magnets producing a combined field strength of 0,08 Tesla at the place of interest.
In an experimental set up the tip prolapse push force on catheters was reduced from 240 to 132 g; the tip curl force (for alignment) was reduced from 45 to 15 g. This indicates how indeed the safety profile of this approach is very promising (2).
The potential to advance and retract the catheter via robotics, or with a simpler system - CardiodriveTM , was also developed by Stereotaxis. This combination permits perfect remote control over the catheter, and should result in less radiation for the physician.
Electrophysiology (EP) studies
It was shown that all basic steps for a diagnostic mapping EP study can be undertaken using the described principle of magnetic navigation. If one wants to combine pacing and recording, a conventional catheter has to be inserted as well (3).
Catheter ablation
Supraventricular tachycardia
We completed our learning curve for AVNRT, an arrhythmia with a simple anatomy in about 20 patients. The results were just as good as with conventional radiofrequency, or cryotherapy (table 1), with considerably less radiation for the patient as well. Due to the magnetic field, less catheter movement with cardiac and respiratory cycles and
Table 1 – Comparison of ablation variables in AVNRT, using 3 different techniques
| Magnetic ablation | Cyrotherapy | RF | |
|---|---|---|---|
|
Number of ablations (median (range)) Total ablation time (s) median (range)) |
6 (1-22) 240 (60-633) |
5 (2-16) 633 (452 - 2599) |
7 (2-28) 290 (90-895) |
| Success | 19/20 | 12/12 | 5/5 |
| Procedure time (min) (median (mean +-sd)) | 163 (167+-46) | 148 (167+-65) | 159 (177+-61) |
| Fluoroscopy time patient (min) (median (mean+-sd) | 12(17+-12) | 17 (20+-11) | 30 (30+-8.8) |
during junctional rhythm were seen than with conventional approaches (4). We have used the system for ectopic and incisional atrial tachycardia and right and left sided accessory pathways. We used a retrograde approach for the latter, even with the present catheters, which are floppy over the entire length. This creates some difficulties at the aortic arch and valve. Others have preferred the transseptal route, which may result in better appositioning of the catheter in the left lateral region.
Ventricular tachycardia
Ventricular tachycardia of non-ischemic origin can be addressed. In RV outflow tract tachycardia, conventional ablation poses some difficulties, as two opposite curves have to be accomplished, and precise mapping has to be performed in a relatively small area. This makes precision movement difficult, and often induces non-relevant arrhythmias. We have approached both left and right-sided idiopathic VT with
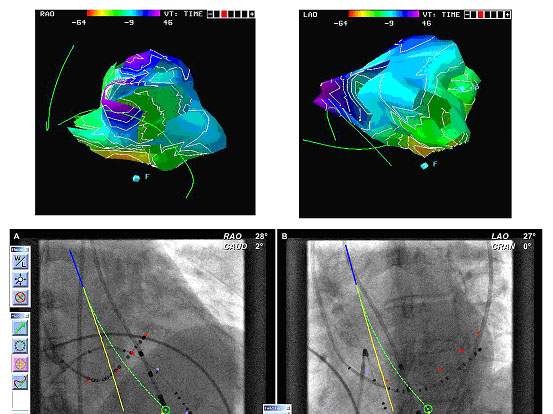
Figure 2. Left ventricular fascicular tachycardia. The upper panel shows the isolines in RAO and LAO, as acquired with the RPM mapping system, showing the apico-septal origin of the arrhythmia, with reference catheters in the coronary sinus and the right ventricular apex. The lower panel shows how the magnetic ablation lead (Helios 2, stereotaxis Inc) is moved from the yellow to the green line by magnetic forces. Here was a fascicular potential, and ablation was performed at this site. As both systems are not integrated, some imagination is left to the researcher. The angulations in RAO are not exactly the same in both views.
magnetic navigation. This allows small steps, and very precise mapping with the floppy magnet catheter.
Atrial fibrillation (AF)
AF is the real challenge today. Some attempts were made by Ernst et al to achieve pulmonary vein isolation. People tend to accept now that a wide circumferential approach is the best way to proceed. Pappone recently presented the first cases with integrated CARTO imaging, resulting in successful ablation of the AF substrate. The perspective should be that even the less invasive retrograde transaortic - transmitral approach should be considered.
Complex congenital heart disease
Corrected complex congenital heart disease is frequently associated with arrhythmias. These can often be very difficult to control with conventional means (5). Reconstructed chambers, recesses, patches, and conduits make catheter manipulation and ablation very difficult. If there is a place for magnetic navigation, it is in these dilated, scarred tissues of operated aging hearts. Integration of anatomy, voltage mapping and activation mapping should assist in more performant ablation.
Advanced cardiac mapping
Electro-anatomic maps to guide the ablation process can now more easily be constructed aided by this remote technology, which ultimately will be automated. This will shorten the procedure time, and assist in assessing the success of otherwise lengthy procedures such as pulmonary vein isolation.
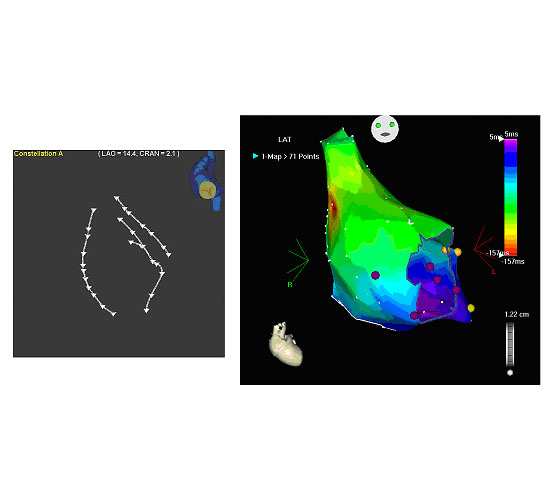
Figure 3. At the left designer lines as suggested by the investigator using the Navigant software (Stereotaxis Inc). At the right the CARTO map (Biosense Webster) showing how these lines contributed to an electroanatomic map in sinus rhythm.
Attempts are now undertaken to merge (old) MRI and multislice CT images in on-line electro-anatomic maps. The overlay seems acceptable. All want to believe that this is progress. Real image integration in the EP domain will be realized when on-line integration of a 3-dimensional image with the online EP data becomes a fact. This will probably be realized with 3D echo, but if developments in X-ray and MRI are fast, these techniques have a chance to be involved as well.
Cardiac resynchronisation therapy (CRT)
Implanting a biventricular pacing lead in the coronary sinus (CS) can be very time consuming. These procedures can require extensive fluoroscopic screening. This is partly due to difficulties in cannulating the CS, and once the guide wire or the lead is in the CS, partly to attempts to reach the potentially best side branch, and finally because the lead has to remain there after it is advanced over the magnetic guide wire, when the guide wire is retracted and the sheath removed. Further, complications as dissection of the CS and pericardiac tamponade exist. The idea to cannulate the CS with a sheath into the mid right atrium or without a sheath at all should be tested with dedicated guide wires.
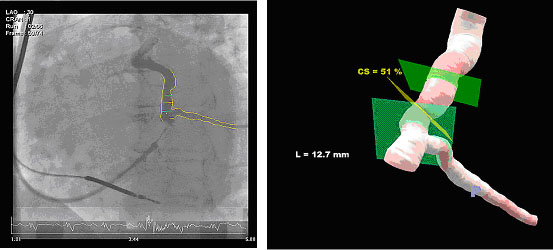
Figure 4. At the right coronary sinus venogram using dedicated software to reconstruct a 3D image (at the left) what can be used to direct the guide wire (3 D vessel reconstruction, PAIEON).
Further, target side branches should be reached with the assistance of magnetic navigation, once the vessel is engaged. Magnetic force should be able to keep the guide wire in position when advancing the pacing wire. Most of these principles were tested already in our lab, and hold great promise (6,7).
Future concepts
It is expected that with the incorporation of catheter registration technology or echocardiography to this system or using these technologies in parallel procedure and radiation times will decrease. Combining this technology with other new technology such as cryotherapy and the other mapping technologies mentioned above, may significantly improve outcome, as well as reducing the number of applications, and associated collateral tissue damage. The potential for other areas in cardiology is there: stem cell therapy, difficult coronary artery procedures, congenital heart disease. Even when this system is only a first step on the road to performant magnetic navigation, the tested principles so far seem to confirm that the concept is valid, and should be considered a milestone in the development of safer and automated procedures.
The content of this article reflects the personal opinion of the author/s and is not necessarily the official position of the European Society of Cardiology.


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.