Introduction
In patients with severe valvular heart disease, guideline-based surgical valve replacement or transcatheter implantation of a prosthetic heart valve is associated with improved survival and relief of symptoms. Prosthetic heart valves are designed to replicate the function of native valves by maintaining unidirectional blood flow and can be separated into two broad categories, mechanical and bioprosthetic (also called tissue) valves, each with different advantages and disadvantages.
This mini-series is divided into 4 parts:
Part 1 – Prosthetic valves: selection
Part 2 – Prosthetic valves: antithrombotic therapy
Part 3 – Prosthetic valves: imaging
Part 4 – Prosthetic valves: complications and dysfunction, pregnancy
Prosthesis-related complications
A range of early or late complications can occur with prosthetic valves depending on the valve type and patient-related factors, including the following:
- Structural failure (bioprosthetic valves)
- Thrombosis and thromboembolism
- Pannus formation
- Paraprosthetic regurgitation
- Intravascular haemolysis
- Endocarditis
Structural valve degeneration
Every valve prosthesis introduces a new disease process, and prosthesis-related complications undermine their attractiveness. While contemporary mechanical heart valves (MHV) are durable, structural valve deterioration (SVD) is a clinically important long-term complication of bioprosthetic valves (BPVs), whether surgical or transcatheter, resulting in a limited life span. SVD is an acquired intrinsic valve abnormality defined as progressive deterioration of the leaflets or supporting structures, resulting in thickening, calcification, tearing, or disruption of the prosthetic valve (PV) with eventual haemodynamic dysfunction, manifesting as stenosis, regurgitation or a combination of both [1,2]. SVD of BPVs results from two not necessarily independent processes, leaflet calcification and non-calcific tissue degeneration.
Clinical factors associated with accelerated calcification include young age, end-stage renal disease, and disorders of the calcium metabolism, thus mirroring some of the risk factors for native aortic valve calcification. A mitral position also confers a higher risk, possibly related to higher mechanical stress on the closed leaflets during ventricular systole as opposed to the lower diastolic pressure acting on a BPV in the aortic position [3,4]. In addition, glutaraldehyde fixation used to reduce allograft immunogenicity and promote collagen linkage for a solid tissue structure is a contributor and potentiates calcification.
The precise mechanisms have not been fully elucidated; the relative contribution of host, mechanical, and valve factors remains unclear [5]. However, the age of the recipient at the time of implantation is the most important determinant of SVD [4], a fact that strongly influences the choice of PV (see Part 1 of this mini series). Several lines of evidence indicate that SVD is not only a degenerative condition, but due to an active disease process at the cellular and molecular levels. SVD is not a binary categorical parameter but a continuous variable, described in a similar fashion to native valve pathology. Moreover, SVD has a deleterious effect on survival after 10 years [3,4]. Currently, there is no pharmacotherapy available to prevent or slow the progression of SVD.
In contrast to prosthesis-patient mismatch (PPM), SVD is a progressive process with gradual haemodynamic deterioration and abnormal valve haemodynamics not present at the time of prosthesis implantation. However, some patients with PVs may already show a somewhat increased gradient at baseline due to borderline PPM which makes the differential diagnosis more difficult. Moreover, Doppler velocity and pressure gradients are flow-dependent, and a mild gradient increase may be related to an increase in transvalvular flow. Therefore, the diagnosis of SVD requires definite structural abnormalities as a key diagnostic feature in addition and proportional to haemodynamic valve dysfunction.
Diagnosis of prosthetic valve dysfunction
Normally functioning PVs have distinctive auscultatory findings. It is important for the clinician to be familiar with normal acoustic phenomena in order to diagnose a PV dysfunction [6]. The motion of the occluder mechanical prosthesis generates very audible opening and closing sounds. In tilting disc and bileaflet valves, the opening click is less prominent than the closing click. A decrease in intensity or even absence of opening or closing sounds with an MHV as a result of hindered disc movement usually signifies severe prosthesis obstruction due to thrombosis or pannus formation. Early systolic sounds do not occur with aortic BPVs. No PV in current use has an effective orifice area (EOA) as large as that of a native valve, and even normally functioning PVs have a pressure gradient across them associated with flow acceleration and a physiologic flow murmur. However, even significant periprosthetic or transvalvular leaks may be inaudible on physical examination. Echocardiography is the primary diagnostic tool for detection of PV dysfunction.
PV obstruction is caused by reduced movement of the PV leaflets due to thrombus, pannus ingrowth, or vegetation. Patients with mechanical valves and to a lesser degree BPVs are at risk of leaflet thrombosis because the coagulation cascade is triggered via the intrinsic system pathways when blood comes in contact with artificial surfaces. MHV thrombosis can be obstructive or non-obstructive and may or may not cause symptoms. Mechanical left-heart valve thrombosis is a potentially life-threatening condition due to severe haemodynamic dysfunction (stenosis or regurgitation through the valve) and may clinically present with dyspnoea, acute pulmonary oedema or thromboembolic events.
Symptom onset can be acute or progressive depending on the rate and degree of valve dysfunction. Occasionally, it may only be an incidental finding during routine imaging. Physical examination may reveal muffled mechanical heart sounds, a new murmur or a change in a pre-existing murmur. No single echocardiographic parameter can accurately assess PV function. A range of normal qualitative, semi-quantitative and quantitative values applicable to most PVs in the aortic and mitral position have been published in the respective societal documents and guidelines [7,8]. These commonly used collective values define three diagnostic categories: (1) normal valve function, (2) possible stenosis in case of intermediate values, and (3) significant obstruction. However, there are no data on how to weigh each parameter hierarchically.
When all parameters are normal, valve dysfunction is unlikely. Conversely, the more parameters are abnormal, the more the probability of PHV obstruction increases. A systematic, integrated and comprehensive approach should be performed after exclusion of technical and measurement errors. Since elevated gradients in PVs can be, but are not necessarily, caused by valve dysfunction, reversible causes of increased flow in case of high-output states (e.g., fever, anaemia, hyperthyroidism, atrioventricular shunts for dialysis) have to be excluded. The mean in contrast to the peak pressure gradient is less flow-dependent and also useful in analogy to native valve stenosis.
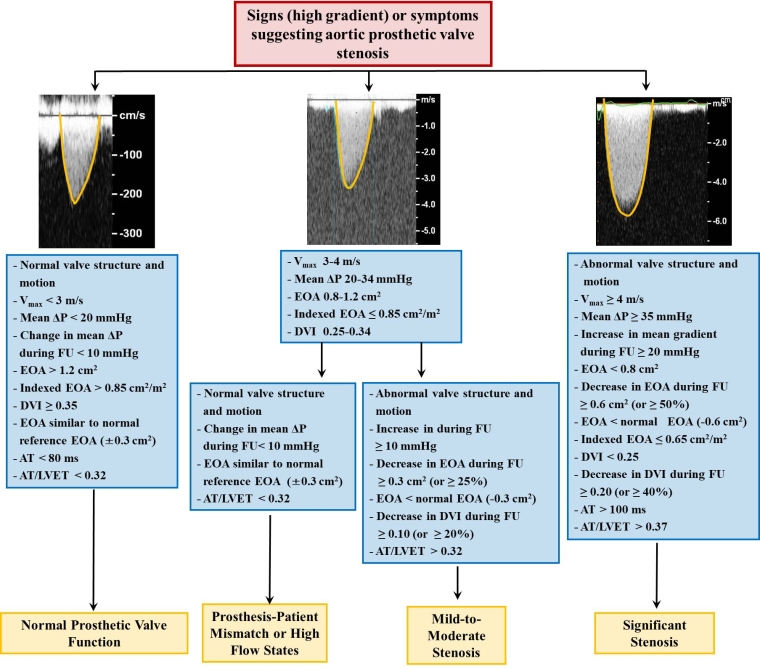
The American Society of Echocardiography (ASE) recommendations define possible aortic BPV stenosis as peak prosthetic aortic jet velocity 3-4 m/s, mean gradient 20-35 mmHg, EOA 0.8-1.2 cm2, and Doppler velocity index (DVI) 0.25-0.29. Significant aortic BPV stenosis was defined as peak prosthetic jet velocity >4 m/s, mean gradient >35 mmHg, EOA <0.8 cm2, and DVI <0.25 [8].
The European Association for Cardiovascular Imaging (EACVI) suggests incorporating an increase in mean gradient at follow-up: possible obstruction with an increase in mean gradient of 10-19 mmHg; significant obstruction with an increase ≥20 mmHg [7]. The American College of Cardiology/American Heart Association (ACC/AHA) guidelines for the management of patients with valvular heart disease suggest a transvalvular mean pressure gradient increase of more than 50% (or >10 mmHg across an aortic valve) compared to baseline as another working definition of PV obstruction [9]. These dynamic definitions emphasise the utility of serial measurements.
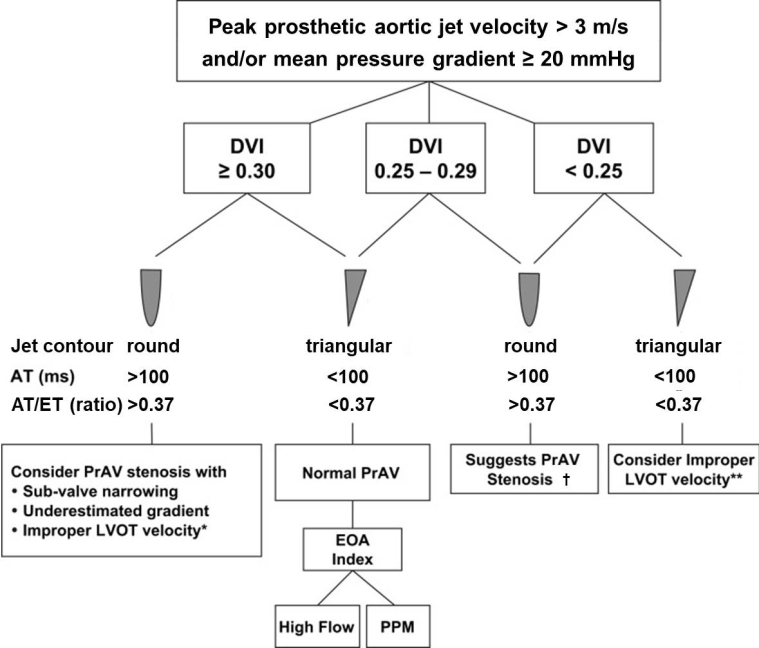
For aortic PVs, a peak velocity >3 m/s should alert the clinician and lead to prompt determination of the DVI and acceleration time (AT). Obstruction becomes likely if the DVI is <0.25 along with a more rounded parabolic (instead of triangular) and late peaking transvalvular continuous-wave Doppler spectrum with an AT >100 ms or >0.37 when indexed to the LV ejection time because the AT is highly dependent on heart rate. These two parameters are also less flow-dependent than transvalvular velocities and also applicable in case of concomitant prosthetic aortic regurgitation (AR) (Figure 1). Typical Doppler findings of an obstructed BPV are displayed in Figure 2.
Figure 1. Algorithm for interpretation of abnormally high transprosthetic pressure gradients in prosthetic aortic valves (PrAV) based on the DVI, contour of the transprosthetic continuous-wave Doppler spectrum, and the acceleration time (AT) in absolute numbers and as the ratio of acceleration time to ejection time (AT/ET).
Adapted and modified from Zoghbi et al [8] with permission.
*pulsed-wave Doppler sample volume too close to the valve. **pulsed-wave Doppler sample volume too far apically from the valve. †calculation of the effective orifice area (EOA) and comparison with reference values for prosthesis type and size recommended. DVI: Doppler velocity index; LVOT: left ventricular outflow tract
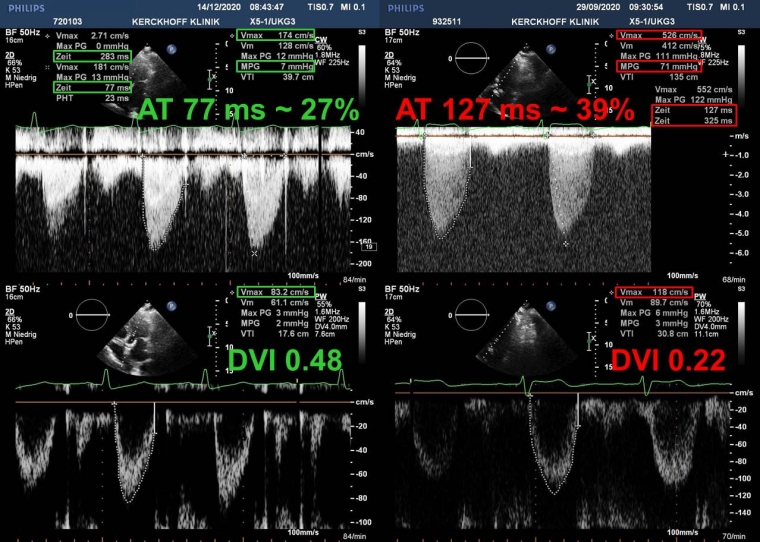
Figure 2. Continuous (top) and pulsed-wave Doppler recordings (bottom) of a normal (left) and degenerated bioprosthetic valve in the aortic position (right).
With prosthetic obstruction, the mean gradient is increased to 71 mmHg (>20) and the Doppler velocity index (DVI) decreased to 0.22 (<0.25). In addition, the acceleration time (AT) from onset of aortic flow to peak velocity is increased to 127 ms (>100), corresponding to 39% (>37) of the ejection time.
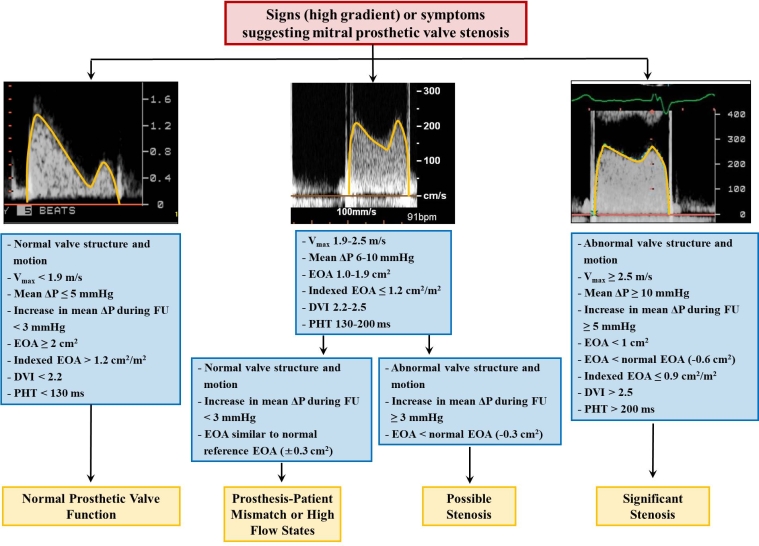
For mitral PVs, a peak transvalvular velocity >2.5 m/s, mean gradient >10 mmHg or pressure half-time (PHT) >200 ms provides a strong argument for significant obstruction. Importantly, the proposed mean pressure gradient is only valid for normal ventricular rates (between 60 and 80 bpm) because an increase in heart rate (HR) is accompanied by a disproportionate decrease in the diastolic filling period and a consecutive increase in the pressure gradient across the mitral valve. For this reason, the HR at which gradients are measured should always be reported. Although the PHT may provide a clue to the presence of mitral prosthetic obstruction, it is not useful for calculating the EOA because this method has only been validated for native mitral valve stenosis. The EOA, however, can be calculated by the continuity equation in the absence of confounders, such as significant AR or MR.
Occasionally, an abnormally high jet gradient is recorded in bileaflet mechanical prostheses regardless of the position (mitral or aortic) because the smaller central orifice jet has a higher velocity than the jets in both larger outside orifices. This phenomenon may lead to underestimation of the EOA and a false suspicion of prosthesis obstruction.
TEE as a complementary tool to TTE allows better visualisation and has a higher sensitivity for identification of SVD, presence of thrombus, pannus or vegetations and is also able to assess leaflet motion better, especially in the mitral and to a lesser degree in the aortic position. Endocarditis should be suspected whenever there is a high clinical suspicion and other more common causes of fever have been excluded. Pannus is an abnormal layer of fibrovascular or granulation tissue which envelopes prostheses. Thrombus tends to be larger and more mobile than pannus and may be associated with thromboembolic complications.
Another distinguishing feature between the two is the lower tissue density of the thrombus compared with pannus. It is important to know that pannus and thrombus may coexist because pannus may induce thrombus formation. On clinical grounds, pannus formation which interferes with normal leaflet movement usually occurs years after valve replacement with a gradual onset of symptoms and is encountered more often in the mitral position. If the diagnosis of valve dysfunction is still in question, the diagnostic work-up should be expanded to include non-echocardiographic imaging techniques, such as cinefluoroscopy and MDCT that can provide valuable information on leaflet mobility. MDCT also aids in the differentiation between thrombus and pannus on the basis of attenuation values. The incidence of pannus formation causing prosthetic obstruction is similar in biological and mechanical prosthetic valves [7]. The following algorithms can be used to interpret high transprosthetic pressure gradients after aortic (Figure 3) and mitral valve replacement (Figure 4).
General approach to prosthetic valve dysfunction
Symptomatic patients with severe prosthetic valve dysfunction represent a phenocopy of the respective native valve disease and should be treated as such according to the societal guidelines [9,10]. Significant stenosis and regurgitation can be found as a mixed lesion on the same prosthetic valve. When either prosthetic stenosis or regurgitation is severe, management should follow the recommendations applicable to the predominant lesion. The combination of a moderate prosthetic stenosis and regurgitation imposes a hybrid pressure and volume overload on the affected cardiac chamber and may be considered differently than either lesion alone. In this setting, the decision to intervene is not well defined by the current guidelines because of limited data. The onset of atrial fibrillation, pulmonary hypertension or right heart failure is indicative of upstream cardiac chamber compromise. Therefore, it is important to consider the objective consequences of such a balanced lesion based not only on the haemodynamic burden but also on the clinical severity. This may include exercise stress testing with measurement of pulmonary artery pressure by either echocardiography or right heart catheter and maximal oxygen consumption for functional assessment.
Bioprosthetic valve thrombosis
Since the first description of bioprosthetic valve thrombosis in a series of cases in 2015 [11], there has been an increasing awareness of this previously unrecognised entity. Hypo-attenuated leaflet thickening (HALT) of aortic bioprosthetic valves associated with reduced leaflet motion (RELM) is the hallmark finding of subclinical leaflet thrombosis using four-dimensional, volume-rendered CT [12]. The hypo-attenuating lesions involve the periphery and base of the leaflet and extend to varying degrees to the edges of the leaflet in the centre of the bioprosthetic valve. TEE is an alternative imaging modality for HALT detection with 100% concordance between the two imaging methods, while this finding is frequently missed by TTE [11]. Paucity of colour Doppler flow by TTE within a region of the BPV in the absence of artefacts may be a clue to underlying HALT in patients with unremarkable transprosthetic gradients.
A recent combined analysis of the two large RESOLVE and SAVORY registries reported a 13% prevalence of this entity observed more frequently in transcatheter than in surgical valves which may reflect differences in techniques and technology [13]. These “anatomical observations” are not necessarily associated with “functional consequences” because the majority of patients with subclinical leaflet thrombosis were haemodynamically silent: an absolute transvalvular mean gradient of more than 20 mmHg or increase greater than 10 mmHg compared to baseline was found in only a small proportion of patients. Although stroke rates were not different between patients with and without HALT, this anomaly was associated with increased rates of transient ischaemic attacks. The precise mechanisms underlying subclinical leaflet thrombosis have not been elucidated. The finding that patients receiving anticoagulation with either non-vitamin K oral anticoagulants (NOACs) or vitamin K antagonists (VKA) compared to antiplatelet agents alone had a lower rate of HALT suggests a thrombotic aetiology. There was also no apparent difference between mono and dual antiplatelet therapy (DAPT). In the absence of another indication, DAPT can thus be considered dispensable in favour of antiplatelet monotherapy.
Other risk factors include a large BPV and sinus of Valsalva, possibly with resultant slower blood flow and stasis in the valve periphery that could contribute to thrombosis [14]. Initiation of anticoagulation with either VKA or NOACs usually results in thrombus resolution and restoration of valve function, suggesting that reduced motion is a result rather than a cause of valve leaflet thrombosis. Currently, only VKA or unfractionated heparin (UFH) are recommended for clinical or subclinical thrombosis of transcatheter and surgical bioprosthetic valves before considering reintervention [9,10]. Despite a protective effect of VKA or NOACs, no preventive pharmacotherapy is routinely recommended because subclinical leaflet thrombosis does not have immediate clinical sequelae in the majority of cases.
However, uncertainty exists about later manifestations including premature SVD with reduced BPV durability. HALT is not limited to the arena of aortic BPVs and has also been described in other BPV locations. Further studies are necessary to establish the scope and long-term clinical impact of this phenomenon.
Treatment of mechanical prosthetic valve obstruction
The management is dictated by the cause of obstruction (pannus versus thrombus) and the severity of symptoms. Severe MHV obstruction caused by pannus requires repeat open heart surgery to remove the pannus or to replace the valve. Treatment options for symptomatic thrombotic obstruction of mechanical valves include intensified antithrombotic therapy, thrombolytics or repeat surgery. MHV patients who have experienced a thromboembolism despite adequate anticoagulation should receive a higher-intensity VKA therapy or the addition of low-dose (75-100 mg) aspirin (Class IIa); however, no direct comparison between these regimens has been performed.
The treatment strategy should be individualised considering, in particular, the bleeding risk [9]. The attitude is somewhat different on the other side of the Atlantic, where the ESC [10] recommends surgery after occurrence of a thromboembolic event in the presence of a large (>10 mm) thrombus (Class IIa). The optimal treatment strategy for non-obstructive clinically silent mechanical prosthetic thrombosis has not been defined.
In contrast, critically ill patients (e.g., those in cardiogenic shock) require emergency treatment. Traditionally, these patients underwent redo valve replacement or simple thrombectomy (Class I). In unsuitable candidates with a high or prohibitive surgical risk, fibrinolytic therapy is an alternative. Doppler echocardiography is the method of choice for serial judgement of the haemodynamic success of thrombolysis. Both types of treatment are high risk. The choice should be made by a heart valve team taking into consideration all available clinical factors as well as local expertise.
Treatment of prosthetic valve regurgitation
Similar to that in native valves, chronic regurgitation in prosthetic valves is frequently tolerated for an extended period of time. When significant valvular or paravalvular prosthetic regurgitation leads to heart failure symptoms or haemolysis with refractory anaemia, redo open heart surgery with either suture repair or repeat valve replacement is the standard of care, despite a somewhat higher risk than the initial surgery [9,10].
In patients with high or prohibitive surgical risk, a transcatheter closure of paravalvular leaks in mechanical as well as bioprosthetic valves is a safe and feasible alternative in experienced centres which obviates the need for repeat open heart surgery. However, inadequate mitral paravalvular leak reduction is associated with a negative impact on symptoms and survival [15].
Only patients with degenerated BPVs and transprosthetic valvular regurgitation are also candidates for transcatheter valve-in-valve (VIV) implantation. The majority of patients with MHVs but not BPVs have some degree of haemolysis, with lactate dehydrogenase levels related to the severity of haemolysis. In patients with intractable haemolytic anaemia attributable to paravalvular regurgitation, refractory to medical therapy and requiring repeated blood transfusions, surgery is indicated. In patients with a high or prohibitive surgical risk, percutaneous repair of paravalvular leak is a reasonable alternative, although there is no conclusive evidence to show a consistent efficacy. Notably, treatment of heart failure symptoms with catheter-based therapy is more successful than is treatment of haemolysis [9,10].
Infective endocarditis
Prosthetic valve endocarditis (PVE) is rare, with an overall annual incidence between 3 and 12 per 1,000 patients. PVE accounts for 10-30% of all cases of infective endocarditis (IE) [16]. Bioprosthetic and mechanical valves are affected equally. Patients with PVE experience a higher mortality risk and risk of post-treatment complications compared to native valve endocarditis, regardless of the treatment strategy adopted.
When compared with native valve IE, prosthetic valve endocarditis is characterised by a lower incidence of vegetations but more paravalvular complications, as manifested by annular abscess (necrotic paravalvular cavity without communication to the cardiovascular lumen), pseudoaneurysm (pulsatile perivalvular cavity communicating with the cardiovascular lumen), fistula (communication between two neighbouring cavities through a perforation) or dehiscence of the prosthesis (separation of the sewing ring from the annular tissue). Late dehiscence is often a sign of IE. A rocking motion of >15° of aortic sewing ring excursion is abnormal and may be a clue to dehiscence of the prosthesis.
In mitral PVs, the typical movement of the posterior annulus leads to apparent rocking but lacks the anatomic gap and colour Doppler flow of a true dehiscence [7,8]. As in native valves, the diagnosis of PVE is based mainly on the results of echocardiography and blood cultures. However, the modified Duke criteria (Table 1) show a lower diagnostic accuracy and should not replace clinical judgement. TTE is recommended as the first-line imaging modality as soon as possible when PVE is suspected, although TTE images are compromised by structural components of the PV and suboptimal assessment of perivalvular complications. Therefore, a complementary TEE should be performed, even with unequivocal findings on TTE, in order to detect local complications.
Three-dimensional echocardiography is a diagnostic innovation increasingly performed along with standard echocardiography. It may provide additional information but is limited by a lower temporal and spatial resolution. Both TEE and TTE can produce false-negative results, highlighting the need for repeat echocardiography within 5-7 days when clinical suspicion remains high.
Alternatively, non-echocardiographic imaging techniques, such as MDCT or 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) computed tomography (CT) as a hybrid method can be employed when echocardiography is inconclusive. MDCT is less affected by shadowing from PV components than echocardiography and may be equivalent or even superior to either mode of echocardiography. While 18F-FDG PET/CT possesses a high sensitivity for identifying infectious processes, the specificity is lower due to false-positive results in patients <3 months after cardiac surgery, with postoperative inflammation around the sewing ring and a number of pathological conditions with non-infectious inflammation, such as active thrombi, soft atherosclerotic plaques or vasculitis. Nuclear imaging with single-photon emission CT/CT (SPECT/CT) using autologous radiolabelled leucocytes is preferred in all situations when enhanced specificity is required.
Table 1. Cardiac imaging findings meeting a major Duke criterion in prosthetic valves.
Echocardiography
-
- Vegetation
- Abscess
- Pseudoaneurysm
- Fistula
- New dehiscence with periprosthetic regurgitation
Cardiac computed tomography
-
- Periprosthetic lesions
Nuclear imaging
-
- Increased activity
Various antibiotic treatment regimens are described in detail in the respective guidelines [16]. Patients with PVE and paravalvular complications are rarely cured by medical treatment alone. Studies examining which valve is better for patients who require operative intervention could not find a difference in long-term outcome between the type of prosthesis (mechanical vs bioprosthetic) implanted [17].
Endocarditis prophylaxis is recommended for all patients with PVs (mechanical, bioprosthetic and transcatheter) undergoing dental procedures (Class IIa). In the absence of randomised controlled trials, IE prophylaxis is reasonable because this group of patients is at higher risk of developing IE because of foreign valve surface material and is also at high risk of experiencing adverse outcomes when affected [16].
Prosthetic valves in pregnancy
The increased haemodynamic burden of pregnancy can lead to heart failure in case of pre-existing PV stenosis, regurgitation, or prosthesis-patient mismatch (PPM). Moreover, normal pregnancy is accompanied by known changes in haemostasis that produce a hypercoagulable state with an increased risk of MHV thrombosis [18]. Indeed, the presence of MHV is a predictor for maternal cardiac complications during pregnancy [19,20]. Therefore, guidelines recommend a baseline TTE under normal loading conditions prior to conception to identify a possible PV dysfunction requiring pre-emptive treatment and serve as a reference in case of suspected valve dysfunction during pregnancy [9].
Mechanical valves offer excellent haemodynamic performance and long-term durability. However, the need for anticoagulation increases maternal and foetal mortality and morbidity, and the risk of major cardiac events during pregnancy is much higher than with BPVs [21]. On the other hand, BPVs in young women are associated with a high risk of SVD, resulting in the risk of going through pregnancy with a dysfunctional valve, and eventually in the inevitable need for re-operation.
An additional consideration for women of childbearing age who contemplate pregnancy is the issue of anticoagulation. Women with MHV require continued anticoagulation throughout pregnancy which poses risks for both the mother and the foetus. The potentially teratogenic VKAs cross the placenta and have a dose-dependent relationship with adverse outcomes, including foetal wastage and embryopathy, most frequently encountered but not limited to the first trimester of pregnancy [19-21]. In contrast, both UFH and low molecular weight heparin (LMWH) do not cross the placenta and embryopathy does not occur. LMWH carries a lower risk of osteoporosis and heparin-induced thrombocytopaenia. Randomised studies comparing the effectiveness of different regimens are not available. The optimal anticoagulation strategy remains controversial and needs to be decided on an individual basis. Besides continuation of VKA throughout the pregnancy, the following options are available: 1) UFH or LMWH throughout pregnancy, or 2) UFH or LMWH for the first trimester, followed by a VKA. Each regimen has drawbacks with regard to maternal or foetal risk. VKA treatment is associated with the lowest risk of maternal adverse outcomes, whereas the use of LMWH throughout pregnancy is associated with the lowest risk of foetal adverse outcomes. However, foetal risk is similar between women taking low doses of VKA and women treated with LMWH. The use of a combined regimen of LMWH and VKA does not appear to have a lower risk of adverse maternal outcomes [22].
Therefore, as per current guidelines, continuation of VKA throughout pregnancy should be considered when the daily warfarin dose is <5 mg (or phenprocoumon <3 mg or acenocoumarol <2 mg) because the risk of embryopathy is low, while VKA are in large series the most effective regimen to prevent valve thrombosis [9,21]. A twice daily dose-adjusted LMWH regimen is superior to a fixed weight-based dose regimen because LMWH is entirely cleared by the kidneys and glomerular filtration rate (and clearance of LMWH) increases during pregnancy. During weekly monitoring, the peak anti-factor Xa levels drawn 4-6 hours post injection should achieve a goal of 0.8-1.2 U/ml for MHVs in the aortic position and 1.0-1.2 U/ml for other locations.
Alternatively, subcutaneous or intravenous fixed or dose-adjusted UFH (activated partial thromboplastin time ≥2 times the control) can be used in high-risk patients. Delivery should be planned and may require transition to UFH and a brief cessation of anticoagulation. There are no efficacy data regarding the addition of antiplatelet agents in pregnant women with MHV on anticoagulation. Prospective randomised studies and large patient registry databases are needed to investigate different approaches of anticoagulant treatment strategies in pregnancy.
Management of MHV thrombosis is comparable with management in non-pregnant patients. Anticoagulation should be optimised in non-critical patients with recent subtherapeutic anticoagulation. Emergency cardiac surgery is indicated in critically ill patients with an obstructive left-sided MHV thrombosis. Although maternal mortality during cardiopulmonary bypass (CPB) is now similar to that in non-pregnant women who undergo comparable cardiac procedures, CPB in pregnant women is associated with poor neonatal outcomes. Fibrinolysis should be considered in critically ill patients when surgery is not immediately available or in non-critically patients when the risk of surgery is high [21].
Despite earlier concerns, recent studies have reported that pregnancy is most likely not a risk factor for accelerated or early SVD requiring premature reintervention [23].