Introduction
The regeneration and reconstitution of an intact endothelial monolayer is essential for the prevention of atherosclerotic lesion formation and progression (1). Until recently, vascular repair mechanisms were thought to be mediated by the adjacent endothelial cells within the vessel wall via proliferation and migration. Recent investigations have demonstrated that circulating bone marrow (BM)-derived endothelial progenitor cells (EPC) play an important role in neoangiogenesis of ischemic tissue and endothelial cell repair after endothelial cell damage(2;3).
Vascular Progenitor Cells and Endothelial Cell Regeneration
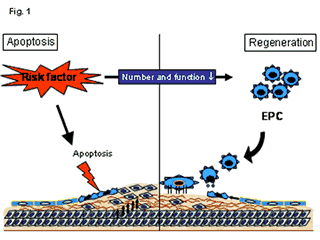
Cardiac risk factors lead to endothelial apoptosis via mechanical (e.g. arterial hypertension) and biochemical (e.g. hyperlipidemia, diabetes) injury of the vessel wall. In addition, cardiac risk factors negatively influence the number and function of EPC. The imbalance between endothelial cell damage and impaired endothelial cell regeneration leads to the formation of endothelial dysfunction and atherosclerotic lesion formation (figure 1). Therefore, a major pre-requisite for the prevention of atherosclerotic lesion formation is an effective regeneration of the endothelial monolayer. Experimental studies have demonstrated, that vasoprotective agents such as estrogens can mobilise EPC into peripheral blood and enhance endothelial cell repair (4). Physical activity -which is known to have great impact in primary prevention- mediates at least parts of its vasculoprotective effects by EPC mobilisation (5). Statin treatment can effectively mobilise vascular progenitor cells into the peripheral blood and enhance the homing process of circulating EPC to the damaged endothelial monolayer (6;7). Finally, the systemic application of EPC after experimentally induced endothelial cell damage enhances reendothelialisation, reduces neointima formation and prevents restenosis (8).
To systematically investigate the vasoprotective role of EPC and vascular progenitor cells in humans, the “Endothelial Progenitor Cells in Patients with Coronary Artery Disease” (EPCAD)-Study was initiated. In this study, the number and function of more than 500 patients with angiographically determined coronary artery disease were determined. Furthermore, several subgroup analyses investigate the interdependence of vascular progenitor cells with physical activity and concomitant drug therapy, endothelial dysfunction and restenosis after coronary intervention and various other influencing factors. The results on cardiovascular morbidity and mortality will provide first insights into the role of EPC as a cellular risk marker. First results of the study will be available by the end of 2004.
EPC differ from endothelial cells in that they have a higher degree of plasticity which means that they can differentiate into various different cells. In vitro Stefanie Dimmeler and co-workers have even shown that these cells can be differentiated into cardiomyocytes. So in this context it becomes obvious, that EPC are not like endothelial cells but have the capacity to differentiate into functional endothelial cells.
EPC which differentiate into endothelial cells show similar characteristics as "normal" endothelial cells as far as we know up to date. Dr. Wassmann from our lab has recently shown that endothelial function improves after EPC transfusion due to an enhancement of NO-synthase activity (paper is currently under review).
Conclusion
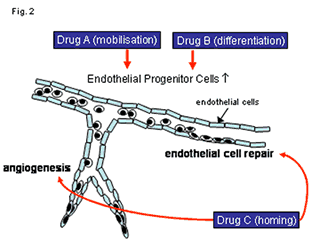
A stem cell based therapy for vascular wall regeneration represents a completely new biological concept with potentially important implications for the treatment of restenosis after coronary interventions and atherosclerosis. The results of the EPCAD-Study will provide a more detailed insight into the role of vascular progenitor cells in patients with coronary artery disease. Future investigations will have to concentrate on therapeutic strategies to improve the mobilisation, differentiation and homing of endogenous stem- and progenitor cells in patients at risk of developing endothelial dysfunction and atherosclerosis (figure 2).
The content of this article reflects the personal opinion of the author/s and is not necessarily the official position of the European Society of Cardiology.



 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.