Introduction
Diagnostic electrophysiological studies and catheter based interventional procedures (ablation) are now facilitated by advanced mapping procedures. However, this mapping still lacks anatomic, realistic information. Virtual cavities can be created, e.g. with the CARTO or EnSite system, or real images obtained with CT or MRI scans, imported and used as a background for electrical signals.
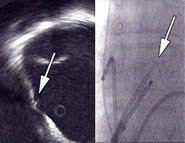
Figure 1. Intra cardiac echocardiogram (ICE) of the right atrium (transversal plane) with the oval fossa and the transseptal needle (arrow). At the right the (frontal) corresponding X-ray is shown, with echo catheter and transseptal sheath with needle.
However, real online images are difficult to obtain with these techniques. One option is to use transesophageal echo; however, this is not possible for lengthy procedures unless general anaesthesia is used. One may overcome this difficulty by using intracardiac echocardiography. This can be done on-line, without the problem of radiation, but still lacks the electrical component. Moreover, echo can be used to facilitate procedures and to avoid complications. It is therefore generally used in the United States, but not in Europe, as reimbursement issues play here a too important role.
Technology today
When the first attempts in the late sixties were made to create a multi-element linear array on a catheter tip to bring it in the heart, the images were not of the quality we see today. However, the experiments showed that it was possible to visualise intracardiac structures (1). Cardiologists had no real interest, or need for intracardiac echo, but were using intravascular probes for coronary interventions. Mechanical transducers have been clinically available since the late nineties, and produced good images of right atrial structures. The commercially available probe (Ultra Ice) is equipped with a rotating motor, and has a 9 MHz crystal (link 1) (2). This is in contrast with the more frequently used IVUS technology (typically 20 MHz), which was developed for the coronary arteries, but can be used on the same platform (Boston Scientific Instruments). The result is a tomography of the transversal plane perpendicular on the catheter. This 360° view with the catheter core in the centre of the image has only little penetration in terms of depth (a radius of 5 cm). This limits the technology to the right side of the heart (figure 1) (2).
Phased array catheters have multiple crystals, are electronically directed and typically scan a sector of 90°. At least two companies have a commercially available product (Siemens / Acunav and EP Med). The depth penetration is better, and the frequency is between 5,5 and 10 MHz (3). This allows a visualisation of left-sided structures from the right side. One advantage of the more recently developed phased array is spectral and color doppler technology. Today, the catheters have sizes of 9 to 10 French. Off line 3D reconstruction of the heart is possible when 2D images are obtained with the ICE catheter using (TomTec). It takes only 5 minutes to do, such that it becomes attractive for clinical practice (figure 2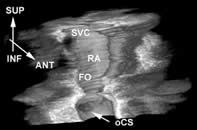
Figure 2. Three dimensional reconstruction of the right atrium (lateral view) with superior vena cava (SVC), oval fossa (FO) and ostium of the coronary sinus (oCS).
Recognition of relevant structures
Well known structures from the pathologist can now be seen directly with echo, where they were invisible with fluoroscopy. The right atrium, which can direct and easily be assessed, contains several relevant structures for E.P. interventions. The best known example is the oval fossa on the interatrial septum, where transeptal puncture is easiest to perform (4). Other visible structures are vessels (coronary arteries, the coronary sinus) and valves (5).
Valves can exist at the os of the coronary sinus, and in the coronary sinus (figure 3) (6).
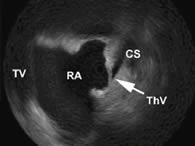
Figure 3. ICE picture showing a thebesian valve (ThV), obstructing the coronary sinus (CS). RA: right atrium, TV: tricuspid valve.
The latter can be an obstacle for diagnostic and pacing catheters). Other valves can be present, as an extension of the Eustachian ridge, and may lie over the entry of the inferior vena cava. Linear structures as the tendon of Todaro, the ligament of Marshall and the terminal crest can be identified as well (7,8). The latter structure is very echo-dense, and plays a role in the pathogenesis of arrhythmias. Some focal (cristal) tachycardias originate in it (sometimes near the sinus node) (9). The crista serves as a functional barrier between vena cava superior and inferior in isthmus dependent flutter (10). Other landmarks are Koch’s triangle, with the compact fibrous body, the septal leaflet and the slow pathway area and the cavo-tricuspid isthmus region. The left atrium is now increasingly explored, and is shaped to a large extent by the pulmonary veins, sometimes with common ostia (11). An important structure is the left atrial appendage. Doppler flow can be used to recognise these structures. Abnormalities in the ventricles (aneurysms, clots) can be seen.
Guiding EP procedures
Transseptal puncture
This technique becomes very easy with echo (link 1). We have experience with both mechanical transducers and phased array. A small fossa can be recognised; tenting can be seen, and contrast may be used to facilitate the puncture (4).
Pacing
Catheter Positioning without fluoroscopy is an option (5). Finding the os of the coronary sinus becomes possible. Atrial septal pacing above the oval fossa shortens the P-wave duration to a larger extent than pacing below the fossa (13). Fluoroscopy is not a reliable tool to assess these differences.
Cardioversion
Screening for clots and spontaneous contrast is as possible with intracardiac echo as it is with transesophageal echo, but has not been validated (14).
Guiding catheter ablation
Atrial flutter and macroreentrant tachycardia
Flutter, also a macroreentrant tachycardia, uses the cavo-tricuspid bridge (the isthmus) in most cases, but occurs as well in post cardiac surgery situations and in congenital heart disease when the circuit is usually around a scar (10). It is necessary to interrupt the conduction at an anatomical acceptable point. The isthmus is highly variable, in shape, length, surface and thickness. This accounts for the failure of some procedures. While we have had difficulties in assessing the isthmus with a femoral approach using the ICE technique, a subclavian route was definitely better in assessing this structure (4). Phased array technology showed to be reliable in the assessment of differences during systole and diastole, and reveals other pitfalls as pits, craters, ridges etc (5).
Furthermore, in congenital heart disease, some malformations in access and structure can be assessed.
It is clear that 3-dimensional echo will be much better than both the actual planar and scalar approaches to judge the cavo-tricuspid isthmus (link 2). Integration of 3D echo with E.P.signals has been realised (still off-line) and has a great future (15).
Atrial fibrillation - Pulmonary vein ablation
Segmental ostial catheter ablation and focal ablation when ectopic activity is present are now becoming widespread approaches for AF ablation. Potentials are only detected when muscular sleeves are seen in the vein, which is feasible with the ICE technology (16). Identification of the left and right upper and lower veins is very easy with a phased array catheter which yields a better vision of the left atrium from the right side (17). Doppler signals can help to identify the atrial appendage. The presence of a common anthrum can be verified. Measurements can be performed, probably with more accuracy than with CT or MR, but certainly better than with venography (17). The position of a lasso catheter can be better judged than with fluoroscopy (18). Encircling of the pulmonary venous ostia can be simplified using an echographic approach (11, 19).
Atrial fibrillation - Linar lesions
With the anatomic and electrical remodelling due to long standing AF, the underlying substrate becomes more important. Linear lesions can play a role in compartimentalisation, and encircling becomes more or less linear. A difficulty inherent to the creation of linear lesions is the need for uninterrupted contact of the catheter with the endocardium, which may be helped by the use of linear catheters.
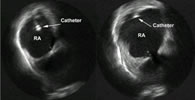
Figure 4. Transversal view in the right atrium (RA) showing how the catheter is not well appositioned to the wall at the left and makes contact at the right (ICE).
Echocardiography can be used with this purpose (figure 4).
Ventricular arrhythmias
Some investigators have used echo to assist in VT ablation, as it allows to see aneurysms, dyskinesia and dysplasia. This can help to avoid complications. That it is helpful in finding the right spot is not shown…. (20)
Monitoring of catheter ablation
Since PV stenosis can be life-threatening, modifications to the procedure have been made, including use of different energy sources, such as irrigated or cooled tip ablation, and cryothermy.
Radiofrequency Continuous monitoring of radiofrequency applications by intracardiac ultrasound has been described (19). Bubble formation is an indication of overheating, and related with emboli.
Cryotherapy Adequate contact between a cryocatheter tip and the endocardium can be assessed. The ice ball is visualised as an hypoechoic zone bordered by a slightly hyperechoic rim. With this technique, no evidence of microcavitation (gas) is observed, and ice ball growth can be monitored over time. Use of echocardiography leads to the observation that applications of 2 minutes are sufficient (21).
Lesion assessment Using myocardial contrast echocardiography (e.g. with an agent such as Sonovue) could be a way to visualise lesions more precisely. We assessed Koch’s triangle before and after ablation of the slow pathway area in atrioventriculonodal reentry, first at baseline, later with the contrast product (figure 5), and could clearly assess the extent of the lesions (both radiofrequency and cryoenergy) (7).
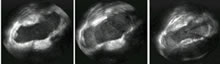
Figure 5. Transversal view in the right atrium (ICE). At the left: basic image, focused at the region of Kochs triangle. Middle: enhancement of myocardium with a contrast agent. At the right : image after ablation, after repeated contrast injection. A clear well-defined laesion is observed.
Prevention and recognition of complications
By using echocardiography, direct assessment of structure and function is possible. This allows to prevent and recognise complications (pericardial effusion, perforation of the aorta, thrombus formation) (22). Doppler has been used to evaluate damage done to the pulmonary vein.
Conclusion
Intracardiac echography is only at the beginning of a bright future. On-line, 3 D images will be integrated in our (radiological and mapping) equipment, and assist in catheter positioning, for ablation and alternate site pacing. This will facilitate procedures, reduce intervention and radiation times, and prevent complications.
The content of this article reflects the personal opinion of the author/s and is not necessarily the official position of the European Society of Cardiology.


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.