Introduction
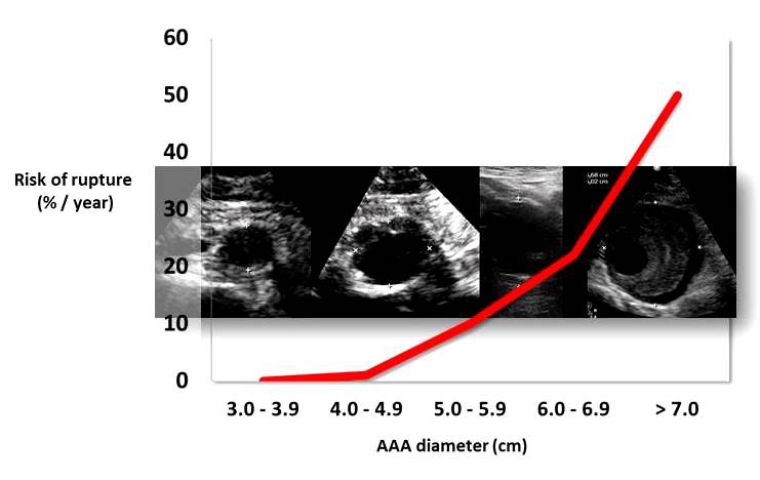
Abdominal aortic aneurysm (AAA) is a segmental, full-thickness dilatation of the abdominal aorta which exceeds the normal vessel diameter by 50%, although a diameter of 3.0 centimetres (cm) is commonly regarded as the threshold. The natural history of AAA is one of continuous expansion and, as it grows larger, so does the risk of rupture (Figure 1) [1].
The established risk factors are advanced age, male sex, smoking and a family history of AAA. It is also important to consider that the risk of having an AAA rises to 10% in patients with peripheral vascular diseases, to 25% in cases where an aneurysm is located in another part of the body, and to 53% if there is the presence of an associated popliteal aneurysm. Diabetes, on the other hand, is associated with lower risk [1,2].
Since AAA is generally asymptomatic, the first manifestation is usually a rupture that escalates into a high mortality situation where between 60% and 80% of the patients die before reaching the hospital [3]. Therefore, a massive screening programme has the possibility of avoiding deaths caused by this pathology, provided that the appropriate treatment is carried out.
Screening
A systematic screening is considered appropriate for a disease when it is a prevalent cause of morbidity and mortality, can be detected in the pre-symptomatic stage by means of effective and innocuous diagnostic tests, and when early treatment has better results than at the symptomatic stage [4].
AAA fulfils many of these criteria, especially when the targeted population is men aged 65 and older. This fact, together with the lengthy asymptomatic period that characterises the disease, makes it possible to detect it by innocuous methods such as ultrasound.
Sometimes AAA can be discovered on abdominal examination; however, because of the retroperitoneal location of the aorta, accuracy is low. Abdominal aortic ultrasound is the primary method used for screening given its high sensitivity (95%) and specificity (100%) for the detection of AAA [2].
Regarding the prevalence of AAA in the population, the initial information comes from four randomised studies aimed at evaluating the usefulness of systematic screening for AAA in the UK, Denmark, and Australia [5-8]. The primary outcome for all trials was AAA-related mortality.
These studies based their screening on populations with the main risk factors associated with AAA, thus, for the most part, men over 65 were invited to participate. In these studies, the prevalence of AAA was 4% in the Viborg study [8], 4.9% in the Multicentre Aneurysm Screening Study (MASS) [5], 7.2% in the Western Australia trial [7] and 7.6% in the Chichester study [6].
Nonetheless, there are limitations in translating these results into current daily practice. Today, the prevalence of AAA has seen a twofold to threefold reduction in several European countries. Therefore, it is appropriate to consider the contemporary evidence from two European countries with national aneurysm screening programmes (UK and Sweden) and the Danish Viborg Vascular (VIVA) trial. The current prevalence in 65-year-old men was 1.7% in the Swedish screening programme [9], 1.3% in the UK National screening programme [10] and 3.3% in the Danish screening programme [11]. This trend is probably the result of risk-factor modification, in particular declining rates of smoking habits [2].
What is the impact of AAA screening on mortality?
Large randomised studies have evaluated the impact of AAA screening in total and in AAA-related mortality. A meta-analysis of all the large AAA screening studies estimated that it is necessary to call 240 men between the ages of 65 and 74 (with 80% attendance, that is 192 ultrasounds) to avoid one death by rupture. In 10 years, for every 10,000 men evaluated, it is estimated that 52 lives would be saved, with the risk of 6 deaths related to the elective therapeutic procedure [12]. Therefore, an abdominal ultrasound performed in men older than 65 years can decrease the risk of death due to aneurysm rupture by more than 50% in the following 13 to 15 years, and the number of lives saved with this strategy exceeds that of deaths related to elective reparation procedures [13].
Results of AAA mortality in the four major screening trials have been summarised in a Cochrane Review. The results showed no significant difference in all-cause mortality between screened and unscreened groups of men or women within three to five years after screening (men, odds ratio [OR] 0.95, 95% confidence interval [CI]: 0.85 to 1.07; women, OR 1.06, 95% CI: 0.93 to 1.21). There was a significant decrease in mortality from AAA in men (OR 0.60, 95% CI: 0.47 to 0.78), but not in women (OR 1.99, 95% CI: 0.36 to 10.88) [3].
When interpreting these results it must be taken into account that these trials were carried out in the last century, when the prevalence of AAA was 4-7% in the men screened and most of the repairs were performed using open surgery. Endovascular aneurysm repair (EVAR) has become the treatment modality in elective repair and, increasingly, in emergency repairs as well. In addition, with more widespread use of diagnostic imaging, the incidental detection rate of AAA is likely to have increased. The life expectancy of the population has also increased.
In such manner, the Swedish nationwide study confirmed the result from the randomised controlled trial in a contemporary population [14]. The introduction of screening was associated with a significant reduction in AAA-specific mortality (mean, 4.0% per year of screening, p=0.020). The number needed to screen and the number needed to operate on to prevent one premature death was 667 and 1.5, respectively. However, this screening programme was highly cost-effective in a contemporary setting [14]. To improve cost-effectiveness, risk scores are being developed to improve detection of patients who would benefit from screening, although to date none has been validated.
Further support for AAA screening comes from the Danish VIVA trial where the effects of a multimodality screening for AAA, peripheral arterial disease and hypertension, and subsequent appropriate prophylactic treatment were assessed. After a median follow-up of 4.4 years (IQR 3.9 – 4.8), the study showed a small but statistically significant reduction in total mortality in the screening group versus the non-screening group (hazard ratio [HR] 0.93, 95% CI: 0.88-0.98; p=0.01) [15].
Finally, in 2017 a meta-analysis was carried out of the longest (≥13 years) follow-up results from randomised controlled trials of AAA screening, which concluded that screening reduces AAA‑related deaths (OR 0.66, 95% CI: 0.47-0.93, p<0.02). A reduction in overall mortality was also shown (HR 0.98, 95% CI: 0.96-0.99, p=0.003) [16]. Besides, men who refuse the offer to be screened have a higher mortality rate for AAA-related and unrelated diseases, highlighting the value of healthcare engagement. The reduction in all-cause mortality in participants who underwent screening programmes, beyond the reduction in AAA-related mortality, may be the effect of a reduction in lifestyle-related cardiovascular risk factors that were addressed when attenders accessed medical care for screening [16].
Considering all these data, both the European Society of Cardiology (ESC) and the European Society for Vascular Surgery (ESVS) guidelines currently recommend population screening for AAA with a single ultrasound scan in all men >65 years of age (Class I, Level A) [1,17].
Likewise, ESC guidelines recommend “opportunistic” ultrasound screening for AAA at the end of any transthoracic echocardiography in those >65 years old [17].
Screening and risk factors for AAA
Smoking is the strongest risk factor for AAA, with an odds ratio of >3 for the association, and higher in women [1]. AAA pathophysiology is characterised by four events: infiltration of the vessel wall by lymphocytes and macrophages; destruction of elastin and collagen in the media and adventitia by proteases, including matrix metalloproteinases; loss of smooth muscle cells with thinning of the media; and neovascularisation [2]. These events are related to a chronic inflammatory process and dysfunction of parenchymal cells central to matrix deposition and repair. Smoking may activate tissue plasminogen activator which induces production of matrix metalloproteinases by macrophages and also disrupts collagen synthesis.
Although the dominant risk factor for AAA is smoking, with a recommended screening strategy targeting all men aged 65 years, there is currently no need for targeting screening based on smoking status [1]. However, the U.S. Preventive Services Task Force (USPSTF) recommends one-time ultrasonography screening for men aged 65–75 years who have ever smoked [18].
Family history of AAA is considered to be associated with more rapid growth of the aneurysm and a higher rupture rate, and rupture may occur at a smaller diameter and at a younger age. Even though the benefit of AAA screening in those with a family history of AAA has not been assessed formally, it is recommended in all men and women aged 50 years and older with a first-degree relative with an AAA [1].
When an aortic aneurysm is identified at any location, assessment of the entire aorta and aortic valve is recommended at baseline and during follow-up (Class I, Level C) [17]. Likewise, because of the high co-existence of AAA with other peripheral aneurysms (iliac, femoral, popliteal), these patients are routinely screened for AAA as well as for other peripheral aneurysms [1,17].
In the case of AAA screening in women there is limited evidence. The only study that included women in the screening protocol was the one carried out in Chichester. There was evidence that the prevalence of AAA in women is six times lower than in men (1.3% vs 7.6%), the size is smaller at the time of diagnosis, and the aneurysm-related mortality occurs at older ages (70% after turning 80 years old). A recent systematic review of publications between 2000 and 2015 indicated that the pooled prevalence of AAA in women over 60 years was 0.7% [6,19]. Based on these data, an AAA screening programme in women should not be considered at this time. Further information is required about the aortic size distribution, definition of an AAA, and harms of screening in women.
Patients with lower extremity artery disease often have other concomitant arterial lesions, including AAA. For this reason, duplex ultrasound screening for AAA should be considered [20].
Table 1 summarises the recommendations about which people should be screened for AAA [1,17,20].
|
Table 1. Patients who need to be screened for AAA.
|
|---|
| Men >65 years old |
|
Men and women aged 50 years and older with a first degree relative with an AAA |
|
Presence of an aortic aneurysm at any location or other peripheral aneurysms (iliac, femoral, popliteal) |
|
Patients with lower extremity artery disease |
|
In women, the need for screening should be analysed on a case-by-case basis. |
|
Opportunistic screening during TTE in those >65 years old |
AAA: abdominal aortic aneurysm; TTE: transthoracic echocardiography
Harms of screening for AAA
The principal harms of screening are associated with an increased rate of elective AAA repair (with its associated risk in morbidity and mortality) and its effects on quality of life. The number of elective repairs increased approximately twofold in persons invited for screening, although this was partially offset by the reduction of emergency AAA repairs and the high mortality associated with ruptures [1,14].
Conclusions
AAA screening programmes have been shown to be effective in reducing aneurysm-related mortality and have become increasingly cost-effective as long-term follow-up increases. With the current evidence, ultrasound screening of AAA is recommended in men older than 65 years. Subgroup analyses suggest that the greatest benefit would be obtained in men aged 65 to 74 years.
Furthermore, screening programmes for AAA could provide an opportunity to improve cardiovascular health through cardiovascular risk assessment, lifestyle changes (diet, smoking habits, and exercise) or high blood pressure treatment among participants who are screened.