Keywords:
digital health, e-Health, m-Health, cardiology, telemedicine, wearables
Introduction
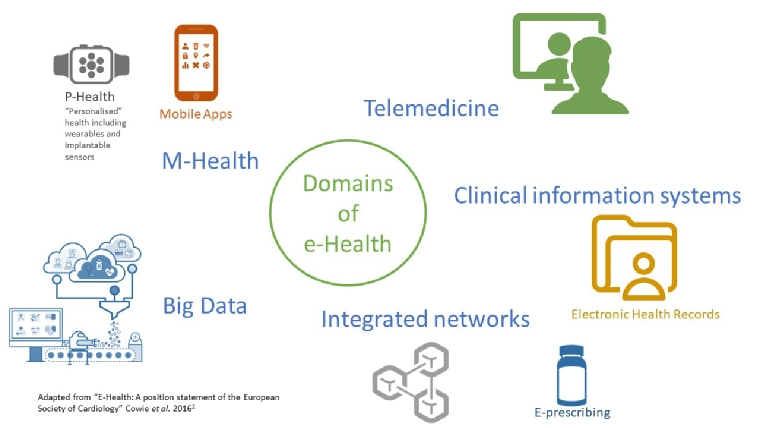
e-Health is defined by the World Health Organization (WHO) as “the use of information and communications technology in support of health and health-related fields” [1]. Ten years ago, this was often limited to the use of computers to view test results, rudimentary electronic health records, or teleconsultation for those living in remote geographies. Today, it encompasses a wide range of uses, from mobile health (m-Health) to telehealth (see Table 1 for definitions), and increasingly underpins all healthcare activity. Figure 1 illustrates the different domains of e-Health [2].
Table 1. Glossary of terms relating to e-Health as per the World Health Organization [1].
|
Term |
WHO definition |
|---|---|
|
e-Health |
The use of information and communications technology in support of health and health-related fields |
|
m-Health |
The use of mobile wireless technologies for health |
|
Digital health |
A broad umbrella term encompassing e-Health (which includes m-Health), as well as emerging areas, such as the use of advanced computing sciences in “big data”, genomics and artificial intelligence |
|
Telehealth |
The use of telecommunications and virtual technology to deliver health care outside of traditional healthcare facilities |
The process of “transformation” of healthcare to be more digital has been accelerated by the current Covid-19 health emergency, which has necessitated much less face-to-face contact and more remote working. A recent review by Eric Topol on preparing the workforce for a digital future outlines the ways technology will change how we provide healthcare: the three key areas identified were telemedicine, smartphone Applications (Apps), and sensors and wearables [3]. Indeed, the smartphone is now a portable (and powerful) personal computer and communications device, with nearly 80% of adults in high-income countries regularly using it to access the internet. The smartphone is arguably the key tool which facilitates m-Health.
This brief review will explore how the digital landscape of e-Health is changing the practice of cardiology and transforming the treatment and prevention of cardiovascular disease in general.
Electronic health records (EHRs)
EHRs are a fundamental component of e-Health. Different EHRs offer varying levels of functionality, from basic documentation to real-time display of clinical signs and observations, often linked to communication with other healthcare professionals and electronic prescribing. EHRs have so far had a mixed reception from clinicians: a survey of US primary care physicians reported that although 63% believed EHRs have generally led to improved care, 74% reported that using an EHR increased their workload, and 68% stated that EHRs took valuable time away from patient care [4]. A time and motion study from the USA showed that 49% of clinician time was spent on EHRs and administration [5].
There is no unified EHR across Europe: even within a single country, individual healthcare organisations typically procure their own software and can be at varying levels of digital maturity. Interestingly, primary care tends to have a more unified approach to EHRs than hospital-based systems. Interoperability is consequently a key challenge: data cannot easily be transferred from one system to another, the coding used may differ, and much of the information may not be easily searchable. Across Europe there is also the issue of language differences.
Currently, there is a major drive politically (backed by patients) to ensure better access of citizens to their own health data, within the constraints of digital security and confidentiality. Ninety percent (90%) of EU citizens expect to be able to access their own health data [6]. The ability to do this is currently limited. Patients may also have healthcare interactions across international borders: the European Union’s (EU) “eHealth Digital Service Infrastructure” (eHDSI) aims to allow cross-border sharing of EHRs, and e-prescription and dispensation of medications by 2021 in 22 EU countries.
For clinicians to be able to make the best use of data from m-Health approaches (including Apps and wearables), the data have to be easily viewed, imported into the EHR, and shared with other relevant healthcare professionals. A clear “audit” trail is required to show on what basis decisions were made.
m-Health
m-Health is the use of mobile wireless technologies for health. This often, but not always, involves the use of a smartphone. A simple use of m-Health is remote access to healthcare information and services. Estonia’s “e-Health record” is an example of a nationwide system whereby patients and emergency services are able to access summary health data remotely via an “e-Patient portal”. The National Health Service in the UK has recently launched the NHS App which allows patients to access their summary medical record (including test results) and book appointments, with further functionality being added rapidly. The European Society of Cardiology produces Apps for healthcare professionals, such as clinical guideline Apps, but also Apps for patients. The “My AF” App allows patients to record symptoms and quality-of-life data, which can be shared with healthcare professionals to add further value to interactions with clinicians.
Patient education is a key factor in improving health outcomes, particularly in cardiovascular disease, with improvements in diet, exercise, smoking cessation and medication compliance helping to optimise the outcome of care and lifestyle choices. Health literacy in Europe is poor: in a study across eight European countries, 47% of participants had low or inadequate health literacy, rising to 61% for those with more than one long-term illness [7]. The NHS recently partnered with Amazon to allow people to access health advice via its voice assistant Alexa, and there is a proliferation of educational Apps aimed at presenting information in a more visual, easy-to-understand manner. Others allow symptom tracking and/or are even able to perform basic triage and provide health advice [8].
Various health authorities are providing guidance on how to assess healthcare Apps, perhaps permitting healthcare professionals to be more confident in using data collected by patients, or even “prescribing” Apps that have the strongest evidence for benefit. The National Institute of Health and Care Excellence (NICE) in England has produced an evidence standards framework for digital technologies to help clinicians evaluate the effectiveness and economic impact of new digital technologies, including Apps. The Food and Drug Administration (FDA) in the USA regulates mobile Apps whose functionality could pose a risk to patient safety if they did not function as intended, but many “health and wellbeing” Apps are careful to avoid making definite medical claims to avoid such regulation. The Catalan government, in association with the ICT Social Health Foundation, has created a public library of health Apps which have been accredited as being fit for purpose, trustworthy for the management of health, secure and credible. Similarly, in the UK, the NHS Apps Library catalogues Apps that have been assessed as safe, secure and meeting technical standards.
Sensors
Sensors are integral to m-Health. A sensor measures a signal and collects data which can be transmitted or recorded for further analysis. This may be as simple as a Bluetooth-enabled scale to measure weight, or as complex as a multiparameter monitoring device. Broadly, sensors can be divided into invasive or non-invasive, the latter including both wearable and non-wearable technologies.
Non-invasive sensors
Bluetooth-enabled “smart” devices allow sensors to connect to smartphones or computers to track data and provide trends. Self-blood pressure monitoring, when combined with antihypertensive medication titration in response to readings, successfully reduces systolic blood pressure compared with usual care [9]. Patient-activated lead-I ECG recorders such as the KardiaMobileTM device (AliveCor, Mountain View, CA, USA) have transformed the diagnosis of atrial fibrillation (AF) and paroxysmal arrhythmia. Whilst previously diagnosis was usually made using Holter monitors, which are often limited by availability, technician analysis time and how long they can be worn, personal lead-I ECG monitors are being increasingly used. The Kardia device has been reported to have a 98.5% sensitivity and 91.4% sensitivity (and was cost-effective) in the community diagnosis of AF [10].
Wearables
Wearable devices (sensors that are externally applied to the body) are increasingly popular consumer products. The most common features of wearable devices are activity monitoring and heart rate monitoring and, whilst these are usually used to track fitness, step counters can be useful adjuncts in cardiac rehabilitation [11] and may provide data for prognostication of heart failure (HF) [12]. Wearable, continuous ECG recording can also be done via patches or vests [13] which are more comfortable, less cumbersome and can monitor for longer than standard Holter monitors. Some “smart” watches, such as the AppleTM Watch (Apple, Cupertino, CA, USA) also have irregular pulse detection algorithms, and lead-I ECG recording capabilities. The AppleTM Heart Study demonstrated that its irregular pulse algorithm had potential for community screening of AF. Over 400,000 patients were recruited, 0.5% had an irregular pulse notification and, of those who had an irregular pulse notification and sent back a 7-day ECG patch recorder, 34% had AF diagnosed by ECG [14]. The study particularly highlights how digital technology has transformed research: there were no study centres and recruitment of a large number of study participants was done rapidly and at relatively low cost.
Invasive sensors
Cardiac implantable electronic devices such as pacemakers and implantable loop recorders are invasive sensors; a computer-based algorithm, rather than a physician, analyses the data in real time. More sophisticated implantable devices can monitor not just cardiac electrograms, but several other physiological variables such as intrathoracic impedance, respiratory rate and sleep apnoea. Proprietary algorithms using such data show promise in identifying patients at short-term risk of HF decompensation and can send an alert to the clinician [15]. A significant increase in pulmonary artery pressure usually precedes HF decompensation and so implantable pulmonary artery pressure monitors can be used to titrate HF therapies and significantly reduce repeat hospitalisation in HF patients with recent hospitalisation [16].
Telehealth, self-care and personalised care
Telehealth (the use of telecommunications and virtual technology to deliver healthcare outside of traditional settings) is not yet widely used in cardiology but the growing pressures on outpatient departments will likely accelerate its deployment. The number of outpatient appointments is rising steadily: in the UK, for example, outpatient appointments have nearly doubled in the past 10 years, with cardiology clinic appointments accounting for more than 3% of all NHS outpatient attendances [17]. Alternatives to face-to-face consultations are therefore required, not only to address limited capacity, but also for patient convenience. Video consultations have so far been well received by patients because of reduced waiting times and travel cost [18], but there are few data on outcomes for specialist appointments for long-term health conditions. Telehealth also has the potential to connect clinicians from different specialties or between primary and secondary care, creating a “virtual Heart Team” and potentially saving unnecessary appointments and supporting more holistic care.
Digital health may also facilitate more expert patient self-management, using data collected in real life to help inform better personal decision making (such as diuretic dosage in heart failure, or improving compliance to antihypertensive medication or exercise rehabilitation programmes) with or without formal decision support algorithms. This is very different from the more traditional approach, where a physician would only use data from validated medical equipment, usually based in a hospital or clinic, and with poor accessibility for the citizen.
Increasingly, sensors are supporting both patient and physician decision making. The implantable pulmonary artery pressure monitoring system (CardioMEMSTM device; Abbott Vascular, Santa Clara, CA, USA) [16] enables patients to record their own pulmonary artery pressure. Their physician can then advise them what to do with their medication and can reinforce compliance with their therapy. In the future, such data could be used to bypass the healthcare team, with decision support software reassuring the patient when their physiology is stable, and giving them an early warning to change therapy, e.g., increase diuretic dosage for a period, when this might be appropriate. Such a potential approach has the advantage of reducing the time delay for a healthcare team to review data and to make decisions, but has to be proven to be safe before regulators will support such m-Health initiatives.
Clinicians are often reluctant to move to more remote working with digital technologies, as it is perceived as organisationally complex, and may not be supported by reimbursement. The Covid-19 crisis has led to a sudden massive swing towards such technologies (with support from insurance companies and reimbursement authorities)., and it is unlikely that after the pandemic that healthcare delivery will remain the same; a good experience of remote consultation and monitoring may persuade both patients and physicians to adopt this into usual practice. Integrating data from remote consultation or monitoring into the EHR can be challenging, but is becoming ever easier.
A good example of how digital technologies are supporting change in care comes from blood-glucose sensors in type 1 diabetes. Newer devices, such as the Freestyle LibreTM (Abbott Laboratories, Abbott Park, IL, USA), allow continuous monitoring of blood glucose with dose adjustments managed by people with diabetes, resulting in fewer hypoglycaemic episodes reported [19] and contact with the healthcare system only as an exception. Blood glucose is a single, easy to understand measurement but it is likely that this principle of self-care based on remotely collected data will move rapidly into other aspects of cardiovascular care, such as systemic blood pressure in hypertension, and pulmonary artery pressure in heart failure.
Barriers to the implementation of e-Health
The EU e-Health action plan 2012-2020 identified several barriers to widespread implementation of e-Health programmes including:
- lack of awareness of, and confidence in e-Health solutions among patients, citizens and healthcare professionals
- lack of interoperability between e-Health solutions
- limited large-scale evidence of the cost-effectiveness of e-Health tools and services
- lack of legal clarity for health and wellbeing mobile applications and the lack of transparency regarding the utilisation of data collected by such applications
- inadequate or fragmented legal frameworks including the lack of reimbursement schemes for e-Health services
- high start-up costs involved in setting up e-Health systems
- regional differences in accessing ICT services, limited access in deprived areas.
Though we are now approaching the end of the time period of this plan, most of these issues remain unresolved. The European Society of Cardiology e-Cardiology working group has emphasised several priorities for addressing these barriers [20]. Patient education programmes are essential to tackle the notion that digital interventions are inferior and also to ensure that patients are able to easily access the intervention; older age, low health literacy and low socioeconomic status are factors associated with lower uptake of digital interventions. Co-design of products with patients is therefore essential for wide uptake, and financial support from healthcare systems or insurers may be necessary to help purchase digital technology for patients with low disposable incomes. Healthcare workflows need to be redesigned to ensure that systems are efficient and that physicians still serve primarily as diagnosticians and educators instead of doing tasks that could instead be automated. European-wide digital health legislation is required to maintain high, uniform standards to ensure that data flows are secure, safe and interoperable.
The ESC has embraced digital transformation: it has a Digital Health Committee and devotes a large amount of space at its annual scientific meeting to digital topics, including innovation in m-Health. An annual Digital Summit was inaugurated in Tallinn, Estonia, in October 2019, and a new journal, the European Heart Journal Digital Health, will launch before the end of 2020. Its mission of education, research and advocacy in cardiovascular disease and prevention can only be achieved by supporting the workforce to adopt more modern approaches to care. Co-design of tools with patients and other stakeholders (from the regulatory, reimbursement and political worlds) will improve decision making and healthcare choices and help to facilitate the digital transformation that is all around us.
Conclusions
All domains of e-Health have developed rapidly in the past decade, with further acceleration in adoption evident during the Covid-19 crisis. Remote teleconsultation is now routine for many cardiologists and is appreciated by patients for its convenience. m-Health technologies, usually based around a smartphone, are rapidly expanding and may be useful in the support of health and lifestyle change, and medical decision making. Linking data streams into the EHR can be challenging, but is improving. Increasingly, patients and citizens are using digital technologies to support their own health and healthcare decision making. Reimbursement has often trailed behind innovation, but has also been forced to support more remote approaches in the recent health crisis. Many barriers exist to the full realisation of the potential benefits of digital health and healthcare, but solutions are being found. Preserving high-quality outcomes and improving the experience of care are vital to long-term success. Cardiology stands at the forefront of such digital disruption.