Introduction
Aortic valve disease, especially aortic stenosis (AS), has an increasing prevalence worldwide, so a good screening tool for the selection of patients being referred for further testing and surgical or interventional procedure is always needed. For many years, the physical examination based mostly on cardiac auscultation represented the most useful bedside diagnostic tools for the diagnosis of these diseases. Unfortunately, expertise and proficiency in auscultation has been diminishing in the modern era, since new technologies have been developed.
Increasingly, over the years, cardiac auscultation has tended to be replaced by sophisticated high technology - electronic stethoscopes, handheld ultrasound or echocardiographic evaluation. Until now, there has been no evidence to support the role of auscultatory devices in increasing the sensitivity of auscultation. There were several studies that showed some benefit of handheld ultrasound (HHU) versus physical examination for the diagnosis of different cardiac diseases, but there are no substantial data concerning the role of HHU in the screening of asymptomatic aortic valve lesions. A systematic review by Stanger et al. showed that the sensitivity and specificity of insonation in identifying AS ranged from 62% to 94% and 85% to 98%, respectively, and that these ranges were similar to auscultation [1].
Compared to echocardiography, auscultation is of low sensitivity (56.6-73%) in detecting aortic valve lesions, but with a high agreement rate between examiners and a high specificity (92-98%) [2,3]. The lowest sensitivity was found in subjects with aortic regurgitation (AR), mild valve lesions, as well as in patients with significant lesions and concomitant left ventricle systolic dysfunction [2]. Regarding the value of the diastolic murmur for the diagnosis of AR, older studies have found a sensitivity of 0% to 38% for mild AR and 60% to 80% for moderate or greater AR [4].
With a certain degree of limitation, cardiac auscultation may have a prognostic value in AS. For 35 years, Bodegard et al have followed up a cohort of apparently healthy middle-aged men, including 23.4% of subjects with systolic ejection murmurs. In patients with a low-grade murmur, there was a 4.7-fold, age-adjusted increase in the risk of aortic valve replacement (AVR) compared to an 89-fold increased risk of AVR for those with increased risk of AVR for those with moderate-grade murmur.
The physical examination has also an important role in the preoperative assessment of patients before non-cardiac surgery. The preoperative screening using cardiac auscultation identified 908 out of 3,997 hip fracture patients with an undiagnosed heart murmur, of whom almost a third were diagnosed by echocardiography with significant aortic stenosis, this finding being important for the further perioperative management [6].
Physical Examination in Valvular Aortic Stenosis
Physical examination often provides the first clue to the presence of AS and helps in assessing the severity of the lesion. Precordial palpation, cardiac auscultation and examination of the carotid pulse are very valuable when evaluating a patient with suspicion of AS.
In patients with isolated AS, the precordial apical thrust is accentuated and initially normal in location. In the left lateral decubitus, a bifid apical impulse is sometimes felt: the first impact comes from the left atrial contraction (corresponding to the presystolic gallop) and the second one from the LV contraction. A low-intensity and/or displaced systolic apical impulse could be a sign of low-flow AS or may be caused by other associated cardiac conditions. A thrill may be palpable over the aortic area in significant AS.
The carotid pulse is characterised in normal subjects by a relatively rapid upstroke and a smooth, more gradual downstroke, interrupted only briefly at the pulse peak. These palpable pulsatile changes in the carotid arterial diameter are virtually identical to the intraluminal pressure pulse. In patients with significant AS, the carotid pulse is weak (“pulsus parvus”), rises slowly and has a delayed systolic peak (“pulsus tardus”). The absence of this finding, particularly in an elderly patient with non-compliant vasculature or in patients in a hyperkinetic state, does not exclude severe AS [7].
Cardiac auscultation reveals many important findings that suggest both the diagnosis and the severity of AS. The most important auscultatory findings in AS are: a normal first sound (S1), a soft and single second sound (S2), the presence of an aortic ejection click and the typical basal systolic murmur.
Normally, the murmur is a systolic ejection murmur with onset a short interval after the S1 and the end before S2. When the valve is still flexible the aortic ejection click precedes the murmur. It is commonly heard in the 2nd right intercostal space (the aortic area), but it could also be audible along the left sternal border in the 3rd and 4th interspaces. The murmur is usually harsh and medium-pitched, so it is audible with either the bell or the diaphragm of the stethoscope. It has a characteristic “diamond shape” in the phonocardiogram. Generally, the murmur is loudest in the aortic area, but in 15% of cases it can be better heard at the apex [8]. The murmur is transmitted well and equally to the carotid arteries and both clavicles. Etchells et al showed that the absence of the transmission of the murmur over the right clavicle effectively rules out AS [9]. The murmur may also radiate to the apex, sometimes with a musical quality due to high frequency vibrations, the so-called Gallavardin phenomenon (usually encountered in degenerative AS).
The presence of three or four associated findings - slow carotid artery upstroke, reduced carotid artery volume, maximal murmur intensity at the second right intercostal space, and reduced or absent second heart sound - effectively rules in AS [9].
The AS murmur has to be differentiated from other conditions that could associate a basal systolic murmurs:
- Functional murmurs are generally faint, medium-pitched, very short (proto-, mid- or late-systolic); the S2 is always normal.
- Patients with hypertension or aortic sclerosis could have a similar harsh, medium-pitched murmur, but with a normal or even loud S2.
- In hypertrophic obstructive cardiomyopathy a systolic ejection murmur is heard along the left sternal border and the apex. Typically the murmur is variable, being amplified by exercise, squatting, Valsalva manoeuvre or administration of vasodilator or positive inotropic drugs.
- Supravalvular aortic stenosis produces most of the signs of valvular stenosis, but the systolic click is absent, the S2 is accentuated and the carotid murmurs are very loud.
- The murmur of pulmonic stenosis has similar intensity, configuration and pitch to AS, but it is loudest in the pulmonary area, the S2 is widely split and the murmur extends beyond the S2.
- A similar murmur to that of pulmonic stenosis can be heard in atrial septal defect, but the S2 is wide and with a fixed split.
When the AS murmur radiates to the apex, there are some hints to differentiate it from mitral regurgitation: the apical impulse is normal in location, the S1 is normal, the murmur starts after the S1, it is not holosystolic and it is not transmitted to the axilla.
Clinical Signs Predictive of Severe Aortic Stenosis
The following signs help in the diagnosis of severe AS (Table 1), but no combination of physical findings has both a high sensitivity and specificity, particularly in asymptomatic patients.
Table 1. Clinical Signs Predictive of Severe Aortic Stenosis.
| Clinical Signs of Severe Aortic Stenosis | Mechanism | Pitfalls |
|---|---|---|
| A loud systolic murmur (grade 4 or greater) | The intensity of the murmur reflects the velocity and turbulence of blood flow across the valve |
↓- LV dysfunction ↑-Hyperdynamic states (anaemia, fever, hyperthyroid) |
| Mid- or late- peaking murmur | It takes longer for blood to eject through a very reduced AV area | Difficult to be assessed clinically, especially in patients with tachycardia, atrial fibrillation, low grade murmur |
| A diminished or absent S2 | The aortic cusps are immobile, so the A2 is faint or even not audible | S2 is increased in associated pulmonary hypertension, other valve heart diseases, hypertensive heart |
| Paradoxical splitting of S2 | LV ejection time is prolonged and AV closes after PV | In LV dysfunction the ejection time does not correlate to the AV area |
| The disappearance of an ejection click | It occurs at the moment of maximal opening of the AV, when the valve is bicuspid, still flexible | Usually it is not audible |
| Presystolic gallop (S4) | Forceful atrial contraction into a hypertrophied, non-compliant left ventricle |
Other causes of LV hypertrophy Absent in atrial fibrillation |
| The “parvus et tardus” carotid pulse | Occlusion of more than 75% of the AV orifice produces a plateau pulse and diminished pulse pressure | The pulse pressure increases in patients with non-compliant vasculature or hyperdynamic states |
AV: aortic valve; A2: aortic valve closer; LV: left ventricle; PV: pulmonary valve; S2: the second heart sound
The Intensity of the Murmur
Haemodynamic studies have shown that the intensity of the murmur reflects the velocity of blood flow across the valve, so a very loud murmur (grade 4 or greater) has a high specificity for severe AS. In one study, the echocardiographically derived peak gradient, mean gradient and aortic valve area were highly predictive for correctly identifying the degree of severity of the murmur (87%, 81%, and 87%, respectively) [10]. Despite the high specificity, the intensity of the murmur has a low sensitivity in diagnosing severe AS, being dependent on the hemodynamic status. In patients with a low left ventricular (LV) ejection fraction or low stroke volume, the murmur’s intensity decreases and in hyperdynamic states it is frequently augmented.
A Mid- or Late-Peaking Murmur
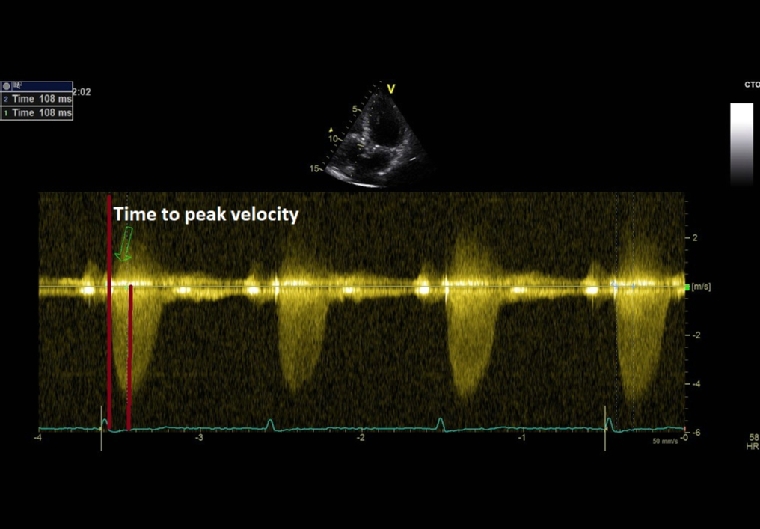
As aortic stenosis worsens, it takes longer for blood to be ejected through the valve. Thus, mild AS would have an early peaking murmur, while in severe AS the murmur peaks later in systole. Even if it is a highly specific clinical sign for severe AS, the evaluation of the timing of the murmur has a very low reproducibility among examiners. Derived from this clinical finding, time-to-peak-velocity (TPV) is a new echocardiographic variable that is easily measured, reproducible and useful to evaluate AS severity (Figure 1). In the study of Kamimura et al, a TPV cut-off value of 99 msec had the highest sensitivity, specificity, and positive and negative predictive values in detecting severe AS (irrespective of ejection fraction) and also for predicting a poor prognosis in AS patients [11].
A Diminished or Absent S2
In severe AS, the aortic cusps are immobile, so the aortic component of the second sound is faint or even not audible. The real proportion of this feature was not extensively studied. In a series of 397 patients with AS at their first haemodynamic evaluation, only 9% had an absent S2 [12].
Paradoxical Splitting of the S2
The paradoxical splitting of S2 is present when the transaortic pressure gradient is very high and the aortic valve closes very late, after the pulmonic valve (more obvious during expiration). Haemodynamic studies showed that, in patients with AS, the direct linear relationship between ejection time and stroke volume is totally obliterated and the degree of prolongation of left ventricular ejection time above that predicted from stroke volume is closely correlated with aortic valve area. On the other hand, in patients with failing ventricles, the ejection time was less prolonged, and the duration of ejection was unrelated to the valve area [13].
The Disappearance of the Ejection Click
The ejection click is a high-pitched sound, best heard at the apex that occurs at the moment of maximal opening of the aortic valve (AV), shortly after the S1. The sound occurs in the presence of a dilated aorta or in the presence of a bicuspid or flexible stenotic AV. It disappears in patients with a severe calcific, immobile aortic valve [8].
The Presence of Presystolic Gallop (The Fourth Sound or So-Called Atrial Gallop)
A prominent S4 can be audible and palpable due to forceful atrial contraction into a hypertrophied, non-compliant left ventricle [8]. The presence of an S4 in a young patient with AS indicates a significant AV lesion, but in an elderly or hypertensive person this is not necessarily true because it can be related to the very common diastolic dysfunction.
The “Parvus et Tardus” Carotid Pulse
Occlusion of more than 75% of the aortic orifice produces a plateau pulse and diminished pulse pressure that can be objectified by carotid or peripheral pulse palpation. Of its two components, “pulsus tardus” is the better discriminator, detecting severe AS with a sensitivity of 31% to 90%, and a specificity of 68% to 93% [14]. A prolonged carotid upstroke time was found in 58% to 68% of patients with severe AS, in 33% of patients with moderate AS and only in 3% of patients with mild AS [15]. A recent study demonstrated that the invasively measured time difference between LV and aortic systolic pressure peaks was significantly associated with the severity of AS and AV calcification calculated on multidetector computed tomography (MDCT) [16].
Physical Examination in Valvular Aortic Regurgitation
In patients with AR, the clinical examination focuses on precordial and peripheral pulse inspection and palpation, as well as cardiac auscultation. The typical early diastolic murmur has a high sensitivity and specificity for the diagnosis of AR (76% and 96%, respectively) [4], though its absence does not exclude the diagnosis. An isolated mid-systolic murmur is a more common auscultatory finding by a non-cardiologist in patients with mild to moderate AR [17].
A displaced (laterally and inferior), diffuse and hyperdynamic apical thrust is a frequent finding in significant chronic AR.
The pulse is characterised by a very brisk upstroke, large amplitude, and rapid collapse (known as Corrigan’s pulse or Watson’s water hammer pulse). It is an extreme form of the hyperkinetic pulse, so we have to differentiate it from other hyperdynamic states [7].
Besides the Corrigan Pulse, there is a long list of classic findings related to wide pulse pressure, but their sensibility and specificity is not well established - dancing carotid arteries (also described by Corrigan), de Musset’s sign (head bobbing with systolic pulse), Mueller’s sign (systolic pulsations of the uvula), Becker’s sign (pulsation of the retinal arteries), Landolfi’s sign (pupillary hippus), Traube’s sign (systolic and diastolic “pistol shot” sounds heard while auscultating the femoral artery), Duroziez’s sign (a systolic and diastolic bruit heard when the femoral artery is partially compressed), Quincke’s pulse (systolic pulsations seen upon light compression of the nail bed) and Hill’s sign (the lower limb systolic blood pressure exceeds the upper limb systolic blood pressure by more than 20 mmHg).
Cardiac auscultation in chronic AR reveals a normal S1, a soft, single or accentuated S2, the typical basal diastolic murmur and a faint mid-systolic murmur.
Normally the murmur is an early diastolic regurgitant murmur that immediately follows the 2nd sound. It is heard in the 2nd right or 3rd left intercostal space (the Erb area), but it could commonly be audible along the left sternal border in the 3rd and 4th interspaces in patients with valvular AR. This is a high-pitched, blowing murmur, best heard with the diaphragm of the stethoscope [8]. Occasionally, the murmur can be musical in quality, the so-called diastolic whoop. It is usually protodiastolic and decrescendo in the phonocardiogram, but in severe AR the murmur is holodiastolic. The murmur is transmitted towards the cardiac apex. The intensity is usually low and does not correlate to the severity of the valve lesion. When faint, the murmur can be better heard end-expiratory or with the patient leaning forward.
A mid-systolic murmur is frequent in isolated AR and is caused either by a large ejection volume through the AV or by calcification of the cusps.
All these findings described in chronic AR may not be present in the acute setting of a severe AR. Usually the murmur with acute AR is a low-pitched early diastolic murmur beginning after the S2, and the signs of acute heart failure dominate the clinical picture.
The AR murmur has to be differentiated from other conditions that could associate basal diastolic murmurs:
- Pulmonic regurgitation is indistinguishable in location, timing and quality from the AR murmur, so the distinction is made only by precordial palpation and the lack or presence of peripheral signs.
- Coronary artery stenosis (Dock’s murmur) is a high-pitched early diastolic murmur, best heard in the 2nd or 3rd left space, near the left border; it is not as widespread as AR.
The following clinical findings suggest a severe AR:
1. A Holodiastolic Murmur
The duration and quality of murmur are directly proportional to the severity of AR. In mild AR the murmur is blowing and only early diastolic. In moderate to severe AR the murmur is holodiastolic (>2/3 diastole) and rougher in quality. However, it becomes very soft or even disappears in patients with LV dysfunction.
2. Marked Peripheral Signs
There are only few data about the predictive value of peripheral signs in diagnosing the severity of AR. Sensitivity reported in the literature for Hill’s sign and Duroziez’s murmur is 69% and 88%, respectively [18]. A difference in blood pressures between lower and upper limb (ankle-brachial difference =ABD) >60 mmHg is highly suggestive of severe AR. In a recent study, the ABD determined by using the cardio ankle vascular index was correlated with LV dimensions and the vena contracta of AR measured by Color echo-Doppler [19].
3. The Austin Flint Murmur
The murmur typically begins in mid-diastole, often has a presystolic accentuation, and terminates at the end of diastole. It is low-pitched, with a rough and rumbling quality, and is best heard at the apex. The proposed mechanisms for the presence of this murmur are fluttering of the anterior mitral leaflet due to the regurgitant jet, increased mitral inflow velocity due to narrowing of the valve orifice by the jet, early mitral valve closure caused by high left ventricular end-diastolic (LVED) pressure, and diastolic mitral regurgitation. The sensitivity reported in various studies is 25% to 100%; the specificity is irrelevant since the Austin Flint murmur is by definition only found in AR [18].
4. Signs of LV Dilation and Dysfunction
The systolic apical impulse is laterally and inferiorly displaced, the intensity of the S1 is decreased (due to the elevated LVED pressure and the early closure of the mitral valve) and a protodiastolic gallop (S3 gallop) is usually heard at the apex.
Conclusion
If we are aware of its limitations and strengths and we succeed in keeping our expertise and proficiency in cardiac auscultation, then clinical examination remains a valuable and cost-effective tool that often enables a rapid, integrative, accurate and patient-orientated diagnosis of aortic valve disease. Although advanced technologies have become part of our daily lives as clinicians, physical examination still plays a crucial role in the screening, diagnosis and evaluation of the severity of aortic valve disease, especially in AS.