Epidemiology of Aortic Valvular Diseases in the World
The epidemiology of aortic valve disease varies enormously between high-income and low-income countries. The majority of morbidity and mortality attributable to aortic valve disease worldwide is due to infectious disease. It may either be directly, as in infective endocarditis, or indirectly, as in acute rheumatic fever (ARF), which is most commonly seen in low-income countries. In high-income countries, the greatest burden of aortic valve disease referred to hospital is due to degenerative calcific aortic valve disease.
The number of cases of aortic valvular disease will increase because of the strong association between valvular disease and age, combined with the rapid ageing of populations worldwide [1]. Valvular disease afflicts the elderly in developed nations, it is insidious in onset, and frequently associated with other comorbidities. However, in low-income countries valvular disease is encountered in the young, not infrequently in children of school age or young females of child-bearing potential, and with a course that is much more rapid. The burden of rheumatic heart disease (RHD) falls disproportionately on low-income countries and in low-income groups in high-income countries. The scope and magnitude of cardiovascular disease are vastly different in different countries (Africa, Asia, Europe or North America).
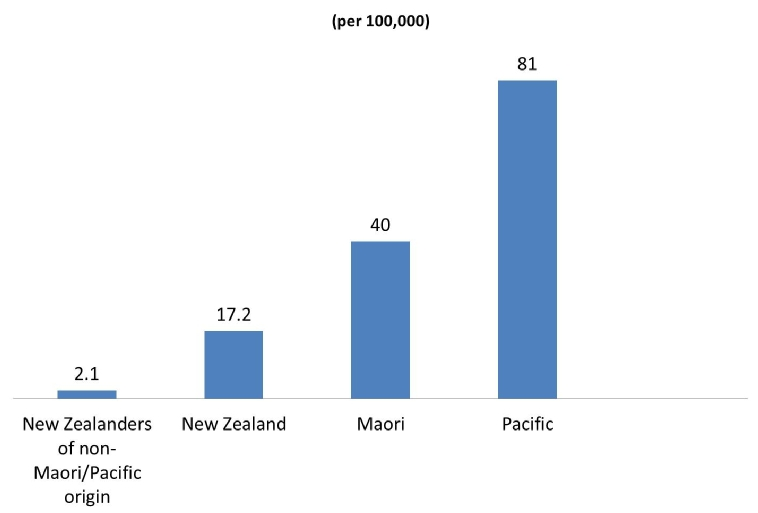
The incidence of acute rheumatic fever has been difficult to establish globally. Estimates range from 10 cases per 100,000 to as high as 374 cases per 100,000 in Pacific and indigenous Australian and New Zealand communities [2]. Indigenous Australians aged 5–14 years (the peak age group to develop ARF have an incidence of 194 per 100,000) [3]. Similarly, in New Zealand, the overall population age-standardised incidence of 17.2 per 100,000 for ARF requiring hospitalisation masks an almost twentyfold increased rate for Maori (40 per 100,000) and a fortyfold increased rate in Pacific people (81 per 100,000) compared with New Zealanders of non-Maori/Pacific origin (2.1 per 100,000) [4] (Figure 1).
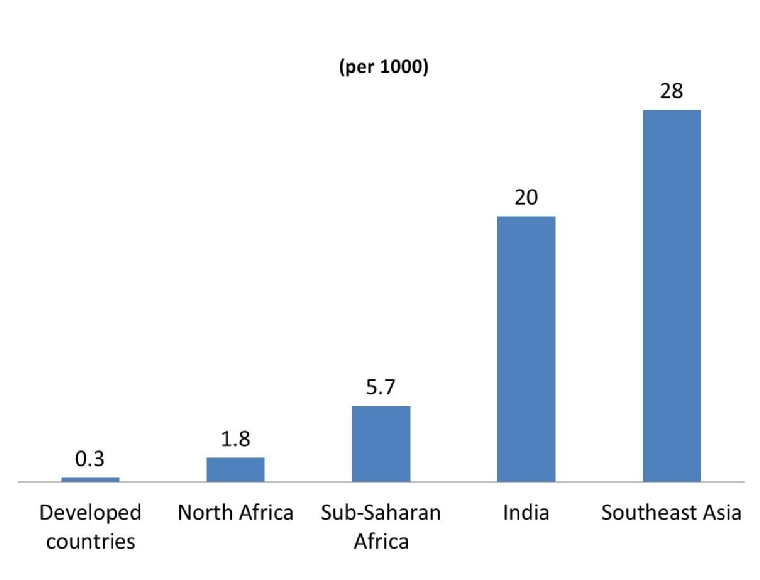
In Africa, ARF is still seen regularly in its most fulminant form, affecting children as young as six years old. It manifests with severe refractory heart failure, often requiring valve replacement in childhood. In the continent of Africa, the World Health Organization expert panel on rheumatic fever (RF) and RHD estimated that in 1994 about 12 million people worldwide had RF and RHD, most of them in developing countries [5]. A more recent review of the current evidence for the global burden of RF and RHD in Africa, estimated that 15.6–19.6 million people have RHD (2.4 million children aged 5–14 years) causing 233,364 – 294,398 deaths from RHD each year (based on an annual mortality rate of 1.5%) [5,6]. Then, the highest prevalence of RHD is in sub-Saharan Africa with a prevalence of 5.7 per 1,000, compared with 1.8 per 1,000 in North Africa, and 0.3 per 1,000 in economically developed countries was established (Figure 2). Recent studies from Africa, using echocardiographic screening, found subclinical RHD in children with a nearly tenfold higher prevalence than without echocardiographic study [7,8].
While previous studies have estimated that the number of children aged 5-14 years with RHD in Asia is between 1.96 and 2.21 million [9], a recent meta-analysis, using echocardiographic diagnosed RHD, estimated that the prevalence of RHD in Southeast Asia was 28 per 1,000 [10]. In another study, the echocardiographic prevalence of RHD in Indian school children was 20 per 1,000 [11]. The fact that the observed prevalence of RHD in India is similar to sub-Saharan Africa suggests that economic betterment may not have translated to improvement in healthcare systems across India. A review of recent studies predominantly using echocardiography for the diagnosis of chronic RHD shows wide global variations in prevalence, between 46 per 100,000 in northern India and 2,400 per 100,000 in the Solomon Islands [12].
Figure 1. Rheumatic Fever Incidence.
Figure 2. Rheumatic Heart Disease Prevalence.
Aortic Stenosis
Aortic stenosis (AS) is, therefore, relatively uncommon in those aged under 65 years in the absence of a congenital abnormality. Aortic stenosis is the second most common valvular lesion in the United States. It is present in about 5% of the population at age 65 with increasing prevalence with advancing age. A meta-analysis of predominantly older studies conducted in Europe, the USA and Taiwan found a population prevalence of AS of 12.4%, and a prevalence of 3.4% of severe AS in those aged 75 years and older [13]. More recent studies have shown relatively similar figures, with 4.3% in an Icelandic cohort aged ≥70 having severe AS [14]. There is an exponential increase in prevalence of AS with age, with 0.2% in the 50–59-year group, 1.3% in the 60–69-year group, 3.9% in of the 70–79-year group, and 9.8% in those aged 80–89 years. The incidence of new AS was 5 per 1,000 per year, with the initial mean age of participants being 60 years.
Information from low-income countries about the burden of calcific aortic valve disease (CAVD) is very limited. Aortic stenosis is uncommonly the predominant valve lesion in RHD (accounting for only 9% of new cases in a large South African cohort) [15]. In younger age groups, clinically significant AS is predominantly due to bicuspid aortic valve disease with tricuspid aortic valve stenosis requiring aortic valve replacement (AVR) only becoming more common over the age of 70 years [16].
A bicuspid aortic valve (BAV) is the most common form of congenital heart disease, being found in approximately 0.5–0.8% of the population [1]. BAV leads to a requirement for treatment at a younger age than tricuspid aortic valves, with a mean age at surgery of just under 50 years. Projections in high-income countries uniformly predict an increase in the burden of disease. Numbers of elderly patients with an indication for treatment (severe symptomatic AS aged 75 years and older), which is the most important group for healthcare systems due to the complexity and cost of management, are projected to more than double by 2050 in both the USA and Europe [13]. Similarly, in Iceland, projections suggest a doubling in prevalence of those with severe AS aged ≥70 years by 2040 and a tripling by 2060 [14]. Once RHD prevalence begins to decrease, CAVD can be expected to play a relatively more important role as a cause of AS in low-income countries. In addition, the mean life expectancy in many low-income countries is currently below the age at which AS due to CAVD becomes clinically significant (e.g., the average life expectancy at birth in Africa was 58 years in 2012) [1]. The high prevalence of risk factors for CAVD such as hypertension and increasing life expectancy, mean that there is likely to be a substantial future burden of CAVD in low-income countries. Epidemiologic studies have established equal rates of AS among men and women; however, important sex differences exist. Women tend to present later in their disease trajectory with a unique risk profile, including older age, frailty, renal insufficiency, and higher rates of symptomatic heart failure (HF) (New York Heart Association [NYHA] Class III–IV), and concomitant moderate to severe mitral regurgitation compared with men [17].
In general, women are also more likely to have smaller annular sizes and left ventricular (LV) outflow tract dimensions associated with concentric LV hypertrophy [17]. Additionally, women have demonstrated a higher prevalence of paradox low-flow/low-gradient AS, which has been associated with poor outcomes and worse mortality compared with high gradient AS [17].
Aortic Regurgitation
Aortic regurgitation (AR) can be due to a primary cause such as bicuspid valve or secondary to aortic root dilatation. AR of mild or greater severity was seen in 13% of men and 8.5% of women in the Framingham Offspring Study [1]. Moderate or more severe AR was estimated to be prevalent in approximately 0.5% of the total USA population [18]. In an African American cohort, 9% had mild AR and 0.5% had moderate or more severe AR [19]. Again, all of these prevalences increase with increasing age. A large multicentre, international registry reported on the sex differences in patients with bicuspid aortic valve: men and women had an equal proportion of each morphology type of bicuspid aortic valve. Men with a bicuspid aortic valve more frequently had moderate/severe aortic regurgitation at first presentation compared with women, whereas women presented more often with moderate/severe aortic stenosis compared with men [17].
Conclusions
In developing countries, aortic valvular stenosis or regurgitation are typically caused by rheumatic heart disease or infective endocarditis. On the other hand, in industrialised countries, valvular diseases are mostly degenerative. Because of the different aetiology in developed nations, aortic valvular disease afflicts the elderly and is frequently associated with other comorbidities. In low-income countries, valvular disease is encountered in the young. The magnitude of cardiovascular disease is vastly different in different countries (Africa, Asia, Europe or North America) and it shows differences according to sex.