Abbreviations
AVSD atrioventricular septal defect
CAD coronary artery disease
DOMV double orifice mitral valve
MR mitral regurgitation
MVP mitral valve prolapse
Introduction
Mitral regurgitation (MR) is the second most frequent indication for valve surgery in Europe. It is essential to distinguish the cause of mitral regurgitation, particularly in relation to management. In primary MR, one or several components of the mitral valve apparatus are directly affected. The common causes of organic (primary) MR include prolapse syndrome, flail leaflet, rheumatic heart disease, coronary artery disease (CAD), infective endocarditis, certain drugs (some anorectic drugs), and collagen vascular disease. In some cases, such as ruptured chordae tendineae, ruptured papillary muscle, or infective endocarditis, MR may be acute and severe. Alternatively, MR may worsen gradually over a prolonged period of time. It is important to know the causes of MR because the management and treatment differ according to the aetiology [1].
Mitral valve prolapse
The most frequent aetiology of mitral incompetence is degenerative (prolapse, flail leaflet). Mitral valve prolapse (MVP) refers to a systolic billowing of one or both mitral leaflets into the left atrium during ventricular systole (valve prolapse of 2 mm or more above the mitral annulus) (Figure 1). The prevalence of this entity is 1% to 2.5% of the population [2]. It is the most common cardiac valvular anomaly in developed countries. Myxomatous degeneration is the main aetiology of prolapsing valvar leaflets, explaining the fact that MVP is uncommon before adolescence. Indeed, the prevalence of MVP was 0.7% in a population of healthy teenagers [3]. In comparison, the Framingham study revealed that 2.4% of adult subjects had an MVP.
MVP occurs as a clinical entity with or without thickening (5 mm or greater, measured during diastasis) and with or without MR. Primary MVP can be familial or non-familial. There is interchordal hooding due to leaflet redundancy that includes both the rough and clear zones of the involved leaflets [4]. The basic microscopic feature of primary prolapse is marked proliferation of the spongiosa, that causes focal interruption of the fibrosa. Secondary effects of the primary MVP syndrome include fibrosis of the surface of the mitral valve leaflets, thinning and/or elongation of the chordae tendineae, and ventricular friction lesions.
Familial MVP is transmitted as an autosomal trait [5,6], and several chromosomal loci have been identified [7-9]. Primary MVP occurs with increased frequency in patients with Marfan syndrome and other connective tissue diseases (Ehlers-Danlos syndrome, osteogenesis imperfecta, dominant cutis laxa or pseudoxanthoma elasticum) [4]. Primary MVP syndrome represents a generalised disease of connective tissue. In patients with MVP there is a gradual progression of MR. Sometimes, spontaneous rupture of MV chordae tendineae or their marked elongation will occur, and patients will develop a flail mitral leaflet. In these cases, valve surgery is indicated because the mortality rate in patients with severe MR caused by flail leaflets is 6% to 7% per year.
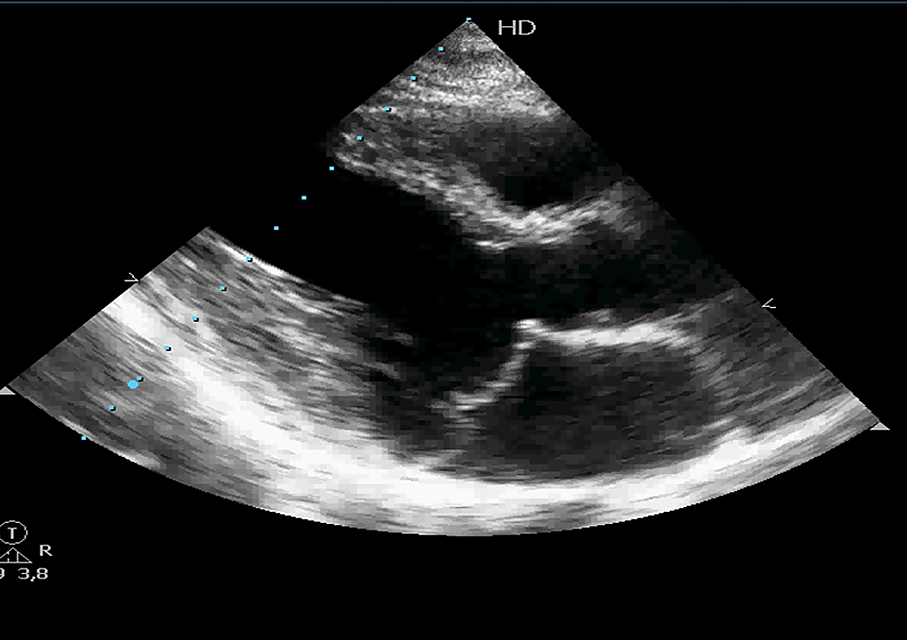
Figure 1. Mitral valve prolapse. Echocardiographic parasternal long-axis view showing both mitral leaflets prolapsing more than 2 mm into the left atrium during ventricular systole, above the mitral annulus.
Rheumatic mitral valve disease
Rheumatic heart disease is the most serious sequela of rheumatic fever, developing to a varying degree in up to 50% of patients with rheumatic fever, leading to mitral valve regurgitation early or later in life [10,11]. It may take several years after an episode of rheumatic fever for valve damage to develop or symptoms to appear. Rheumatic heart disease remains a major cause of morbidity and mortality in children and young adults in the developing world with a peak age group of 25 to 35 years [12]. It is less prevalent in developed countries (0.05/1,000 in the USA) due to improved living conditions, better health care, and availability of antibiotics, but an increasing incidence has been reported in the last two decades in developed countries [3-7].
Rheumatic fever occurs in 3% to 4% of untreated group A streptococcal pharyngitis [8]. This occurs as a result of an autoimmune response against streptococcal antigens that develop cross-recognition to the human cardiac tissue. This mimicry between streptococcal antigens and heart tissue proteins, combined with the production of proinflammatory cytokines and reduced production of interleukin 4, leads to the development of cardiac tissue damage [10,11]. Rheumatic heart disease affects the mitral valve in up to 50% of cases and results in mitral insufficiency, mitral stenosis, or both. In young patients, MR is predominant, but mitral stenosis becomes progressively more common with age. Regurgitant rheumatic valves are oedematous with fibrous thickening and minimal calcification, non-fused commissures, annular dilatation, and anterior chordal elongation.
Mitral regurgitation secondary to coronary artery disease
Papillary muscle rupture is another cause of acute mitral regurgitation. It is a rare, life-threatening, post-myocardial infarction mechanical complication. It may occur 2-7 days after acute myocardial infarction. The rupture may be complete or involve one or more of the heads and is 6-12 times more frequent in the posteromedial papillary muscle because of its single artery blood supply [13]. This specific clinical entity will be dealt with in a later paper in this series (“Ischaemic MR”).
Congenital malformations of the mitral valve
Mitral incompetence may occur as congenital malformations of the mitral valve. They are often complex and affect multiple segments of the valve apparatus. These may occur in isolation or in association with other congenital heart defects. In an echocardiographic study, congenital malformations of the mitral valve were detected in almost 0.5% of the 13,400 subjects [14].
Isolated cleft of the anterior mitral valve leaflet is a rare but well-known finding, the origin of which is under debate. Indeed, some authors have considered isolated cleft to be a 'forme fruste' of atrioventricular septal defect (AVSD), whereas others have supposed it to be a distinct morphological entity.
The definition of a mitral cleft is a division of one of the leaflets (usually the anterior leaflet) of the mitral valve. This must not be confused with the so-called “cleft” in AVSD. AVSD is characterised by a five-leaflet valve guarding a common atrioventricular junction: superior bridging leaflet; inferior bridging leaflet; left mural leaflet; right inferior leaflet; and right anterosuperior leaflet. AVSD can be separated into complete and partial forms, depending on the degree of attachment of the superior and inferior bridging leaflets to the crest of the ventricular septum and to the inferior rim of the atrial septum. In complete AVSD, there is a single common orifice. The partial form is also defined by a common valve annulus, but with the existence of two separate orifices due to a tongue of tissue joining the free margins of the superior and inferior bridging leaflets [15].
More rarely, isolated cleft may be seen in the posterior leaflet of the mitral valve [16]. Although it may occur at any segment of the posterior leaflet, the predominant localisation of the cleft is within scallop P2. Cleft of the posterior mitral leaflet has been reported in association with counter-clockwise malrotation of the papillary muscles that may, again, lead one to suspect a common embryological origin with AVSD.
Double orifice mitral valve (DOMV) is a rare condition occurring in 1% of autopsied cases of congenital heart disease. DOMV is rarely isolated but usually an ancillary finding in the setting of a more complex congenital cardiac anomaly.
DOMV is defined as a single fibrous annulus with two orifices opening into the left ventricle. It differs from duplicate mitral valve, which is defined as two mitral valve annuli and valves, each with its own set of leaflets, commissures, chordae and papillary muscles. Mitral insufficiency occurred in 43% of cases, mitral stenosis in 13% and both stenosis and insufficiency in 6.5% [17].
Although parachute mitral valve is more responsible for mitral stenosis, mitral regurgitation may occur, and it must be equally carefully followed because of its progressive evolution. True parachute mitral valve is characterised by unifocal attachment of the mitral valve chordae to a single (or fused) papillary muscle. This single papillary muscle is usually centrally placed and receives all chordae from both mitral valve leaflets. In parachute-like asymmetric mitral valve, chordae are distributed unequally between two identifiable papillary muscles, with most or all of the chordae converging on a dominant papillary muscle [18]. The dominant papillary muscle, classically posteromedial, is of normal size, whereas the other is elongated and displaced higher in the ventricle with its tip reaching to the annulus.
In both parachute mitral valve and parachute-like asymmetric mitral valve, the chordae are short and thickened, thus restricting the motion of the leaflets. Oosthoek et al [18] assumed that PMV results from an embryological disturbance during the normal delamination of the trabecular ridge between the fifth and nineteenth week of gestation. In this hypothesis, the embryonic predecessors of the normal papillary muscles, derived from the anterior and posterior parts of the trabecular ridge, would condense into a single muscle.
Endocarditis
Endocarditis is one of the causes of primary MR. Mitral valve regurgitation may occur as a result of mitral chordal rupture, leaflet rupture (flail leaflet), leaflet perforation or interference of the vegetation mass with leaflet closure. A particular situation is infection of the anterior mitral leaflet secondary to an infected regurgitant jet of a primary aortic infective endocarditis [19]. Resultant aneurysm formation on the atrial side of the mitral leaflet may later lead to mitral perforation. Urgent surgery is indicated in patients with acute severe MR because it leads to heart failure, which is the most frequent complication of endocarditis.
Uncontrolled infection may cause perivalvular extension of endocarditis with abscess formation, pseudoaneurysms and fistulae, frequently associated with very severe valvular and perivalvular damage. Perivalvular abscesses in mitral endocarditis are usually located posteriorly or laterally [20].
Other rare causes of mitral regurgitation
Prolonged use of certain medications can cause mitral valve regurgitation, such as those containing ergotamine (Cafergot, Migergot) that are used to treat migraines and other conditions. In rare cases, radiation therapy for cancer that is focused on the chest area can lead to mitral valve regurgitation. Experiencing trauma, such as in a car accident, can lead to acute mitral valve regurgitation which can sometimes be life-threatening.
Conclusions
Mitral regurgitation (MR) is the second most frequent indication for valve surgery. Mitral regurgitation may occur for organic or functional causes. The common causes of organic (primary) MR include prolapse syndrome, flail leaflet, rheumatic heart disease, CAD, infective endocarditis, certain drugs, and collagen vascular disease. MR may also occur secondary (functional) to a dilated annulus from dilatation of the left ventricle (from an imbalance between closing and tethering forces) or left atrial enlargement in patients with atrial fibrillation. MR may be acute and severe. Alternatively, MR may worsen gradually over a prolonged period of time and surgery may be necessary by way of mitral valve replacement or repair.















