Introduction
Mitral valve prolapse (MVP) is the most frequent valvular abnormality (prevalence 2-3%) typified by left atrial displacement of either or both of the anterior and posterior leaflets [1]. Although it is usually benign without any serious long-term consequences, in some patients it can result in severe mitral regurgitation, and is associated with heart failure syndrome, arrhythmia and sudden cardiac death [2, 3]. In the majority of cases, MVP is isolated and not associated with other conditions. However, MVP can also be seen as part of other clinical syndromes such as connective tissue disorders and idiopathic dilated cardiomyopathy. A familial pattern is seen in approximately 35-50% of cases, suggesting a heritable component which has prompted genetic research into this condition.
Given that MV prolapse is prevalent and rare events, such as sudden death, have been studied at post-mortem, previously undiagnosed MV prolapse has been noted at a higher frequency in these decedents, suggesting an association [4]. However, this association of MVP with sudden cardiac death (SCD) may in part be due to ascertainment bias. More recent data have identified a plausible link with arrhythmogenicity and a familial predisposition [2, 3]. There are complex variables involved including structural, pathological, electrophysiological and possibly genetic aspects to consider. In this review, we summarise the current understanding of the genetics of MVP and the clinical relevance and impact of these findings.
MVP aetiology and pathophysiology
MVP is defined on imaging by billowing of any portion of the mitral leaflets (≥2 mm above the annular plane on a long-axis view [parasternal or apical 3-chamber]) into the left atrium during systole [5]. The mechanism of prolapse varies and can include disruption or elongation of the leaflets directly or the subvalvular apparatus (chordae or papillary muscles).
Two main forms of MVP exist based on aetiology:
- primary (degenerative disease in the absence of identifiable connective tissue disease and fibroelastic disease); and
- secondary MVP (associated with an identifiable disorder such as connective tissue disease, e.g., Marfan syndrome and Ehlers-Danlos syndrome).
Younger patients tend to present with severe myxomatous degeneration and, in the classic case of Barlow’s disease, bileaflet thickening and redundancy [6]. Older populations present with fibroelastic deficiency disease, in which lack of connective tissue leads to chordal rupture. Discerning these can have important consequences for the management of the disease [7]. It is important to bear in mind that there are overlaps and spectrums of disease; Barlow’s disease also presents in the elderly.
Epidemiology
The epidemiology was initially highly variable in the literature, due in part to poor study design and less stringent definitions of MVP, but is currently estimated at 2.4% (17 million in the USA and 178 million worldwide) [1]. The majority of MVP is benign, but it is associated with varying degrees of regurgitation and with endocarditis, heart failure syndrome and SCD. MVP remains the leading indication for surgery of the MV [7]. MVP exists in sporadic and familial forms, recognised since the 1960s, and has a prevalence of 5-20% in first-degree relatives of probands [8-10].
Electrocardiographic abnormalities
Abnormalities of the T-waves have been reported in cases of MVP and sudden death or ventricular arrhythmias [2]. These repolarisation abnormalities can reflect endocardial and mid-myocardial changes of papillary muscles or the left ventricle and may result in a repolarisation gradient across the myocardium, resulting in T-wave inversion.
Sudden death risk and MVP
Sudden death was observed in 6 (2.5%) patients in an echocardiographic cohort of 237 patients followed for a mean of 6.2 years (1,469 patient-years), where the only variable associated with SCD was redundant leaflets [8].
Cases of sudden death (some with documented ventricular arrhythmias) were noted to have prolonged QT intervals, particularly among female decedents [4, 11]. In a retrospective review of 24 cases with out-of-hospital cardiac arrests, 10 (42%) had bileaflet MVP and 90% of victims were female [3]. These cases had biphasic and inverted T-waves in the inferior leads on 12-lead ECG and a high frequency of premature ventricular contractions (PVC) on Holter monitoring with an origin from the right ventricular outflow tract, papillary muscle, or were of fascicular origin. In a study by Basso et al, imaging and post-mortem findings in patients with MVP and sudden death were compared to patients with MVP and documented ventricular arrhythmias and controls [2]. The study identified scarring which may have been as a result of increased tension in the chordae which transmit to the subvalvular apparatus, resulting in fibrosis acting as an abnormal substrate. Others have proposed possible mechanical triggers as a result of redundant valve tissue prolapsing to the atrium in systole and ventricle in diastole, increasing the propensity for premature ventricular contractions (PVCs), which may initiate ventricular arrhythmias.
Thus, the presence of T-wave inversion, PVCs, fibrosis on cardiac MRI and a family history of sudden unexplained deaths should raise suspicion of a malignant phenotype.
Genetics
Although familial forms have been recognised for some time, determining the genetic aetiology has been challenging until more recent technological advances. Cascade clinical screening of first-degree relatives is highly variable, especially if there are non-syndromic vs. syndromic associations. Familial forms with classic Mendelian inheritance were detected with moderate success using linkage disequilibrium analyses, followed by candidate gene approaches. More recently, novel variant discovery, using whole exome and whole genome sequencing approaches, has been used to identify single genes as well as single-nucleotide polymorphisms (SNP) through genome-wide association studies (GWAS). The current known genes and SNP are described below and classified into syndromic and non-syndromic forms (Figure 1).
Figure 1. Schematic of the complexities of MVP genetics.
The implicated genes have been organised into those associated with syndromic conditions and a higher frequency of MVP than that seen in the general population, those associated with familial non-syndromic forms, and genome-wide association studies.
The red arrow on the apical 4-chamber 2-dimensional echo tracing in late systole shows the posterior mitral valve leaflet prolapsing into the left atrium.
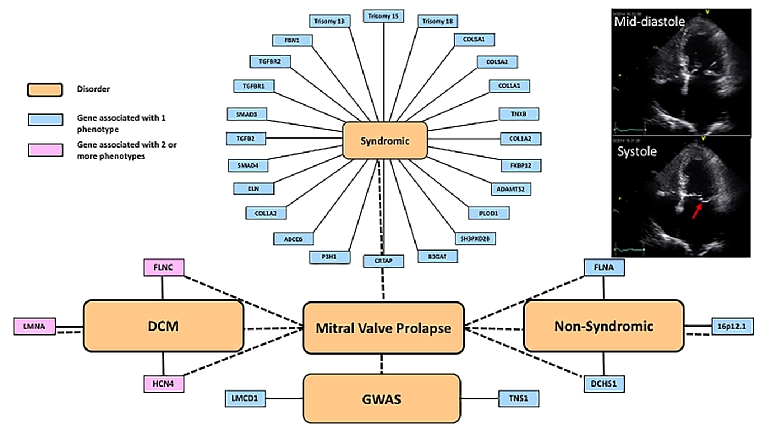
Syndromic and without connective tissue disease
Trisomies (chromosomes 18, 13, and 15)
Trisomies of chromosome 18 (Edwards syndrome), chromosome 13 (Patau syndrome) and chromosome 15 are associated with multiple cardiac defects, which can include MVP, typified by myxomatous leaflets, elongated chordae tendineae and papillary muscle hypoplasia. Most of the trisomies are pleiotropic with marked and severe non-cardiac defects such as cognitive impairment and growth retardation.
Syndromic associated with connective tissue disease
Marfan syndrome (MFS)
Autosomal dominant condition with a prevalence of 1 in 5,000 due to mutations to FBN1 result in type I Marfan syndrome (MFS) which has a high prevalence of MVP (over 75% at age 60 years) [12]. In fact, MVP, detected by an abnormal mid-systolic click on auscultation, can be the presenting feature leading to a diagnosis of MFS; 13% of Marfan patients are referred for MV surgery. The FBN1 gene encodes for the protein fibrillin-1 which binds other fibrillin-1 proteins to form thread-like filaments termed microfibrils which provide strength and flexibility to connective tissue. These microfibrils also normally bind to growth factors – abnormalities in FBN1 lead to excess availability of unbound growth factors which lead to instability of connective tissue throughout the body and contribute to phenotypic heterogeneity and pleiotropy. A diagnosis of MFS is based on the revised Ghent criteria, which includes MVP as a criterion [13].
Loeys-Dietz syndrome (LDS)
A connective tissue disorder of unknown prevalence, initially considered an overlapping Marfanoid syndrome, now recognised as a distinct disease with 5 different types [14]. Like MFS, inheritance is autosomal dominant with phenotypic heterogeneity and pleiotropy. Approximately 75% of cases are sporadic. Five genes have been identified to date, which lead to the 5 types: type I due to transforming growth factor (TGF)-β receptor 1 (TGFBR1); type II due to TGFBR2; type III due to SMAD3; type IV due to TGFB2 mutations; and type V due to TGFB3. In a study comparing the clinical phenotype of FBN1 mutation carriers to TGFBR2, MV prolapse was less frequent in LDS (21% of TGFBR2 vs. 45% of FBN1) and less likely to warrant surgical treatment. Type III due to SMAD3 is also known as aneurysm-osteoarthritis syndrome and MV abnormalities are seen in 50% of patients [15].
Ehlers-Danlos syndrome (EDS)
Ehlers-Danlos syndrome (EDS) is a group of disorders which can be autosomal dominant and recessive and has a prevalence of 1 in 5,000, with various evolving classifications used over the past few decades, as knowledge has increased. There are currently 19 different genes and 13 different types [16]. The cardiovascular manifestations are highly variable and the prevalence of MVP is approximately 6%. Classic EDS is due to mutations in COL5A1 or COL5A2 or COL1A1 and TNXB; non-classic forms include COL1A2, ADAMTS2, FKBP14, PLOD1 [16]. COL3A1 is associated with vascular abnormalities.
Williams-Beuren syndrome (WBS)
Williams-Beuren syndrome (WBS) has a prevalence of 1 in 10,000 and is due to deletions of chromosome 7. This region contains the ELN gene. The ELN gene encodes the protein tropoelastin which forms the mature protein elastin, a major component of elastic fibres, found in connective tissue and the extracellular matrix. Supravalvular and pulmonary stenosis are more frequent findings (45-75%) rather than MVP (6%) [17].
Pseudoxanthoma elasticum
Pseudoxanthoma elasticum is an autosomal recessive disorder affecting 1 in 50,000 individuals with a female:male preponderance of 2:1. A defect in the ABCC6 gene results in widespread organ involvement, classically the skin, eyes, and blood vessels, including arteriosclerosis. MVP is an infrequent finding in approximately 4.5% [18].
Osteogenesis imperfecta
Osteogenesis imperfecta (“brittle bone” disease) in the majority of probands is autosomal dominant and sporadic with a prevalence of 1 in 20,000 live births [19]. There are 8 recognised types, with type I being the mildest and type II being the most severe. There are 4 known genes: COL1A1, COL1A2, CRTAP, and P3H1 which cause osteogenesis imperfecta, although 90% of cases are due to COL1A1 and COL1A2. MVP has been reported, but clear associations remain to be determined. Aortic root dilatation and problems with the aortic valve apparatus are far more frequent.
Larsen-like syndrome
Larsen-like syndrome is a disorder glycosylation with defects identified in the B3GAT gene encoding for glucuronosyltransferase-I (GlcAT-I). The prevalence is unknown and the phenotype includes joint dislocations, bicuspid aortic valves and MVP [20].
Borrone dermatio-cardio-skeletal syndrome
Borrone dermatio-cardio-skeletal (also known as Frank-Ter Haar) syndrome. Autosomal recessive disorder of unknown prevalence with skin, cardiac and skeletal problems. Mutations in the SH3PXD2B gene which encodes for a protein necessary for podosome formation which is involved in cell adhesion and migration [21]. Alternative splicing of the gene results in multiple transcript variants. The gene is also known as FAD49 and FTHS. The frequency of MV prolapse is unknown.
Non-syndromic myxomatous MVP
In addition to the disproportionately high observed prevalence of MVP in syndromic genetic conditions, isolated familial forms of MVP have also been recognised. The first published report suggested X-linked recessive inheritance with only male phenotypic expression in a 3-generational family with polyvalvular disease [22]. Since then, familial studies have suggested 60% heritability, with notable age- and sex-dependent phenotypic expression and reduced penetrance. These studies suggested autosomal dominant inheritance, with linkage studies identifying 3 loci: MMVP1, MMVP2 and MMVP3 described below.
MMVP1 was identified through linkage study mapping to chromosome 16p12.1-p11.2, although the gene and protein remain elusive [23].
MMVP2 in a 5-generation family mapped to locus 11p15.4, following which a missense variant (p.R2513H) in the DCHS1 gene was discovered in the same family. This variant was found in an unrelated family who also expressed MVP. The gene product is a signal peptide belonging to the cadherin family with loss of function impairing cell polarity during valve development in mice [24].
MMVP3 was identified in a 3-generation family with MVP and linkage to chromosome 13q31.3-q32.1 identified [25]. The gene and protein have not at present been identified.
In the family with X-linked inheritance, there was a P637Q mutation in the FLNA gene [26]. More recently, families with muscular dystrophy and MVP have been described. The type of MV prolapse is unusual and may be unique to FLNA-related MVP: myxomatous leaflets with paradoxical restriction of leaflet motion in diastole.
Cardiomyopathies and ion channelopathies (FLNC, LMNA, HCN4)
The genetics of dilated cardiomyopathy (DCM) are complicated with wide phenotypic expression [27]. Certain genotypes have been associated with arrhythmias and DCM. More recently, left ventricular non-compaction (LVNC) and sinus node dysfunction with MVP have been described in persons with HCN4 mutations [28]. The FLNC gene encodes for gamma filamin which crosslinks actin filaments – defects in FLNC cause skeletal myopathy and have recently been recognised as causing arrhythmias and DCM. The frequency of MVP and the association with SCD, MVP and cardiomyopathy-related genes are unknown.
Genome-wide association studies
A GWAS of non-syndromic MVP cases and controls recently identified 6 genome-wide significant loci [29]. Of these, two candidate genes were discussed: TNS1 on Chr2 and LMCD1 on Chr3. Both genes have plausibility with a role in valve development, although further genomic and functional studies are still missing to address fully their implication in the genetic risk of MVP, and to identify causal genes in the remaining associated loci.
LMCD1 encodes a transcription factor repressor of GATA6 [30], an important regulator of cardiac development. Morpholino knockdown of the zebrafish orthologue resulted in regurgitation of the atrioventricular valves, although the exact mechanism of the genetic association with MVP risk is still unknown and could be either through a primary valve defect or as a consequence of a defect in cardiac development.
TNS1 encodes for tensin 1, which is an actin-binding protein. This association strengths the biological importance of cytoskeleton integrity in MVP aetiology, given the enlarged posterior valves described in the mouse lacking this gene. The association described is located on a putative regulatory enhancer of TNS1. Further experiments, specifically on gene regulation at this genetic location in mitral valve cells, will provide support to the role of TNS1 as a mediator of important interactions between actin and focal adhesion proteins during cell migration.
Translational outlook
Although syndromic MVP has been well recognised for decades and the role of genetics is more established, the evaluation is usually based on the syndrome and not the MVP itself. Most syndromic forms are autosomal dominant and a minimum 3-generational pedigree, with evaluation at follow-up (to identify new individuals who may not have expressed the phenotype) is standard in most centres. Although familial patterns and heritability have long been recognised, the more recent genetic elucidation of non-syndromic MVP is challenging understanding of the disease. We advocate a 3-generational family history in cases of MVP and clinical cascade screening of first-degree relatives. This is likely to be cost-effective and easily translatable as MVP (defined above) can be identified. The challenge with mild prolapse not meeting current imaging criteria is more demanding; we suggest avoiding this at the present time. We do not advocate cascade genetic screening for non-syndromic MVP at this time, due to limited understanding and data on the validity, reliability and cost-effectiveness.
The widespread availability of clinical and research genetic testing, including commercial kits available direct to the public without a request from a clinician, is likely to result in variants being identified more readily. Interpreting these genetic findings is a challenge for clinical staff and thus enrolment into clinical research studies with access to experts is recommended. We also advocate that genetic testing without full genetic counselling, including implications of test results, should be avoided.
Conclusions
Understanding the genetic aetiology of non-syndromic MVP vs. syndromic MVP has only just begun. The aims are to improve mechanistic understanding, particularly with regard to the pathogenesis, which may explain the varied phenotypic expression and ultimately pave the way for novel therapeutics. Although the genetics of MVP are still largely in the research domain, the key to better understanding is good phenotyping (with robust echocardiographic assessment) and clinical evaluation for associated features. Furthermore, full and accurate pedigree analysis is key to improving the accuracy of epidemiological data, as well as to understanding heritability. There is a recognised association with sudden death and the presence of T-wave inversion. PVCs and fibrosis on late gadolinium enhancement should alert the clinician that the patient may be at risk of malignant arrhythmias and sudden death.















