Background
Data show the clinical relevance of a condition that Harvey Kemp in 1973 labeled Cardiac Syndrome X (CSX) - angina, myocardial ischemic signs and normal or nearly normal coronaries on angiogram characterised this condition (1). Its pathological mechanisms are epicardial coronary spasm, coronary bridging, coronary slow-flow, Tako-tsubo and coronary microvascular dysfunction (2). Research of cardiac syndrome X has focused on microvascular dysfunction (CMVD), a common pathophysiological dysfunction of this condition together with an altered perception of pain, and especially on its role in the genesis of ischemia.
New methods for assessing microcirculation are also being explored. Indeed, microvascular dysfunction causes microvascular angina; it requires the absence of demonstrable obstructive coronary artery disease (CAD) and an altered microvascular response to functional testing. It is primary, if microvascular angina is the unique cause of symptoms, and secondary, if it occurs in the setting of a specific disease (3). Stable microvascular angina (MVA) has recurrent effort angina, while instable or acute MVA presents as acute coronary syndrome, rest pain and in some cases an increase in mild myocardionecrosis markers (4).
The 2013 ESC Guidelines on management of stable coronary arteries disease (SCAD) (5) dedicate specific consideration to angina with normal coronary arteries, and identify two clinical patterns as part of stable coronary disease: microvascular angina and vasospastic angina - which may involve in some cases only the microvascular district.
1 - Pathogenesis
Coronary microcirculation is not usually the object of routine imaging however, being the major determinant of vascular resistance - 80% of total resistance is due to coronary microcirculation - its dysfunction may compromise myocardial perfusion. Indeed, the coronary pre-arterioles and arterioles - i.e. the coronary arteries <500 μm in diameter, physiologically modulate coronary blood flow (CBF) in response to neural, mechanical and metabolic factors (4-7);
Possible causes for microvascular dysfunction are:
- Cardiovascular risk factors (CVRF) are identified in several studies - particularly hypertension and diabetes as important because they interfere with the normal functioning of microcirculation as well as the epicardial coronary arteries.
- Impaired vasodilatation capacity accompanied in some subjects with enhanced vasoconstrictor tone increase CBF resistance, reduce coronary flow reserve (CFR) and may cause myocardial ischemia (8).
- Endothelium-dependent mechanisms, revealed by failure of CBF to increase in response to acetylcholina or cold stressor test physiologically act by endothelium nitric oxide release (9); Endothelium-independent mechanisms, highlighted by the reduced CBF response to adenosine or dypiridamole, determine smooth muscle cell relaxation (10).
Various further observed alterations can combine in a variety of ways in individual patients and thus, determine the broad clinical spectrum observed in clinical practice of cardiac blood flow impairment:
- Administration of vasoconstrictor stimuli, such as acetylcholine or ergonovine (2) caused altered vasoconstrictor response and is related to enhanced vasoconstriction induced by endothelina-1 release.
- Microvascular spasm and ST-segment depression was associated with severe chest pain in the absence of epicardial spasm (11). In these patients, even physical exercise seems to induce vasoconstriction, rather than vasodilatation (12).
- Rest slow coronary flow, suggestive of basal microvascular constriction, is also described (13).
- Reduced vasodilators’ response to effort may explain positive effort angina and positive stress tests by causing ischemia in distal areas and a blood steal phenomenon by normal microvessels.
- Symptoms at rest may be related to enhancement of vasoconstrictor tone in response to stimuli such as acetylcholine or cold pressor test and to increased basal vascular tone, suggested by coronary slow flow
- Metabolic evidence of myocardial ischemia during stress tests has also been reported (4).
On examination, myocardial ischemia is detected by stress ECG or Holter's dynamic monitoring, which show ST-segment depression and chest pain, but not by stress-echography, because the patchy distribution of perfusion abnormalities makes the abnormalities macroscopically undetectable. Ischemic areas may be pointed out by stress scintigraphy, which show stress induced perfusion defects, by magnetic resonance (MR) or positron emission tomography (PET).
Here are areas of study investigating the causes of CMVD:
- Common cardiovascular risk factors (CVRF) - e.g., hypertension, hyperlipidemia, diabetes mellitus, smoking - may lead to CMVD (3). The risk profile of a subject with MVA is similar to the one of a subject with obstructive CAD (14), but the reason why some subjects develop epicardial artery disease whereas others develop microcirculation disease is unclear.
- Disautonomic imbalance can also explain the altered vasomotor control in microcirculation: increased cardiac adrenergic activity, resulting in an enhanced adrenergic drive (15) or reduced parasympathetic tone (16), has been detected in 75% of patients with CSX, and it seems confirmed by enhanced arterial pressure and heart rate response to effort and low variability of heart rate in these subjects.
- Abnormal pain perception is also investigated: the abnormal pre-arterioles constriction might cause the release of so much adenosine to provoke angina despite poor metabolic or functional signs of myocardial ischemia (17).
- Increased insulin resistance, estrogen deficiency (in women), enhanced activity of the membrane sodium-hydrogen exchanger, and subclinical inflammation Other suggested causes are (4).
Several studies investigate the association between cardiovascular risk factors and CMVD in women and their results are discordant:
- The presence of cardiovascular risk factors cannot reliably predict neither endothelium-dependent nor endothelium-independent microvascular disfunction: Indeed, cardiac syndrome X patients have a lower occurrence of all conventional CVRF compared to patients with obstructive CAD (18) - while others have a similar risk profile.
- Atherosclerosis risk factors poorly predict abnormal coronary microvascular reactivity to adenosine, suggesting that other as yet unidentified factors must primarily account for CMVD (19).
- Another study confirms these findings in women and identifies epicardial fat thickness as the only independent predictor of microvascular dysfunction (20).
Nevertheless, mounting data show that CVRF can affect microcirculation and are associated with CMVD, suggesting that they do so in at least a proportion of patients.
- In diabetic and hypertensive subjects without epicardial coronary stenosis, CFR impairment is demonstrable.
- In one study, this is partly explained by increased left-ventricular mass, which in turn conditions the hyperemic stimulation of myocardial blood flow (21),
- Another study doesn’t point out any significant difference between hypertrophic and non hypertrophic hypertensive hearts in terms of microvascular functionality (22).
- If compared to non diabetic patients with other similar cardiovascular risk factors, diabetic patients without significant CAD most often show impairment of coronary microvascular function, associated with normal myocardial perfusion (23). They have greater involvement of microcirculation than hypertensive population (24).
In hypertensive subjects especially:
- Microvascular functionality indices (such as Timi frame Count and Myocardial Blush Grade) are more impaired compared with those of normotensive subjects;
- A positive nuclear imaging, show a partially reversible hypoperfused area, as severe as worse angiographic indices are relieved (22).
- Impaired coronary reserve correlates with evidence of transient ischemic episodes in the Holter's dynamic monitoring, whereas neither left ventricular hypertrophy nor arterial pressure are determinants for ST-segment depressions (25).
In obese subjects with evidence of neither heart disease nor epicardial artery stenosis,:
- CFR is lower than in lean subjects, and IL-6 and TNF-α are the only determinants of CFR (26).
- Compared with asymptomatic obese subjects with comparable cardiovascular risk factor profile, obese subject with CSX, have impaired brachial artery flow-mediated dilatation, that is significantly associated with elevated high sensitivity-CRP concentrations and BMI, supporting the concept that obesity and low-grade inflammation mediated by adipocytokines may promote vascular dysfunction in these patients (27).
The role of inflammation is also demonstrated in CSX patients without traditional cardiovascular risk factors: subjects with elevated CRP levels have significantly reduced CFR, compared to those with low PCR levels (28).
2 - Assessment
Microcirculation can't be investigated by angiogram, thus, several techniques for functional assessment, of coronary flow reserve,both invasive and non-invasive, have been proposed (29, 30) (Table 1). Microvascular function is evaluated by testing vascular flow responses to vasodilators stimuli using various techniques, and calculating Coronary Flow Reserve (CFR) as the ratio between hyperemic and basal coronary flow. The most widely used substances are adenosine and dipyridamole, to test endothelium-independent vasodilatation, acetylcholine (ACH) and cold pressure to the endothelium-dependent one. A CFR below 2.5 (although a threshold of 2.0 might be more specific and diagnostic) is usually considered as diagnostic of CMVD impairment. However, non-invasive techniques may lack sensitivity and specificity for diagnosis and are unable to differentiate between epicardial and microvascular abnormalities. A complete characterisation of coronary microvascular (CMV) function would also require, especially when vasodilator tests are normal or inconclusive, the assessment of response to vasoconstrictor stimuli (ACH, ergonovine), performable only during an invasive study, with the aim of exclusion of significant vasoconstriction of epicardial vessels and detection of microvessel spasm (4).
Non invasive methods - Transthoracic Doppler echocardiography (TTDE) is the first screening test to identify significant impairment of CMV function. It allows the measurement of CBF velocity in the left anterior descending artery. CFR is defined as the ratio of hyperemic diastolic peak flow velocity during maximal vasodilatation to basal flow velocity. Results are comparable with those obtained by intracoronary Doppler flow wire (ICDW) recording and PET. Myocardial perfusion and CFR are also assessable through cardiac MR, myocardial contrast, echocardiography and PET. These tests each have their own limitations in terms of ability in perfusion quantification, required expertise, availability, costs and potential risks; thus, they are second line methods in patients in whom TTDE is inconclusive or in whom assessment for specific aims is required (29).
Invasive methods - during angiography it is possible to assess CMV function through several techniques: thermodilution or gas washout method and intracoronary Doppler (ICD) flow wire allow CBF and CFR quantification; intravascular Doppler ultrasonography (IVDUS) permits the direct visualisation of arterial walls and detection of atherosclerotic plaques that foregoes to the angiography. is the most used is the intracoronary recording of CBF by Doppler and pressure wires.Intracoronary Doppler is the most used techniques as it allows direct measurement of CBF velocity in single epicardial arteries. The product of CBF velocity and the cross-sectional arterial area gives a measure of CBF. Evaluations prior and post vasodilators infusion allows the CFR measurement. Through intracoronary Doppler and pressure sensor incorporated, it is possible to calculate the Index of Microvascular Resistance (IMR), defined as the distal coronary pressure multiplied by the hyperaemic mTT.
Angiogram allows microvascular function evaluation indirectly through some angiographic indexes.
Myocardial blush is the myocardial opacification resulting by injection of dye into the coronary. Counting the number of heart cycles required for it to fade out, we achieve the Myocardial Blush Grade (MBG), which depends on the microcirculation resistance to the dye passage and the efficiency of venous drainage (32). Total Myocardial Blush Score is the sum of the MBG of each coronary territory and defines the overall microvascular functionality (33).
The Timi Frame Count (TFC) is calculated on the basis of the number of frames required for dye to reach a standardized distal landmark of the considered coronary vessel and a correction factor depending on vessel length; it is related to the velocity wherewith dye fulfill epicardial vessels and index of microvascular district resistance (34). Similarly to the Total Myocardial Blush score, Total Timi Frame Count, is the sum of the three major coronary vessel scores, useful for a comprehensive view of the coronary microcirculation function (35).
Table 1. Comparative summary of methods to investigate coronary microvascular function (29).
| Availability | Costs | Risks | Repeatability | Operator dependancy | Full CMV Function assessment | Quantitative CBF measure | |
|---|---|---|---|---|---|---|---|
| TTDE | +++ | +++ | +++ | +++ | - | - | - |
| MCE | ++ | ++ | + | ++ | +/- | + | +/- |
| PET | - | - | +/- | +/- | ++ | +++ | ++ |
| CMR | +/- | - | + | +/- | ++ | +++ | ++ |
| ICD | +/- | - | +/- | - | + | + | + |
CBF, coronary blood flow; CMV, coronary microvascular; CMR, cardiac magnetic resonance; ICD, intracoronary Doppler; MCE, myocardial contrast echocardiography; TTDE, transthoracic Doppler echocardiography. - Poor for the item; + sufficient for the item; ++ good for the item; +++ very good for the item.
3 - Clinical scenarios
A finding of angina with a normal arteriogram may occur in the setting of a chronic (stable) or an acute (unstable) angina syndrome (4). In both cases, the first step in diagnosis is to differentiate from non-cardiac chest pain.
Chronic microvascular angina - About 50% of coronary angiograms in patients with stable angina show normal or non-obstructed (<50% stenotic) coronary arteries. This condition is much more common in women than in men, and may be due to vasospastic angina (Prinzmetal's variant angina and microvascular spasm) (36), or to chronic microvessels disease, either isolated or associated with systemic (amyloidosis, Fabry) or cardiac disease other than CAD.
Chronic MVA is characterised by association with classical atherosclerotic risk factors, history of recurrent angina, often typical and mainly effort induced that require repeated diagnostic evaluation with non-invasive stress tests (frequently with abnormal results) and even coronary angiography.
Differential diagnosis on clinical features is impossible, but some clues suggest MVA: chest pain persists for several minutes after effort interruption and/or shows poor or slow response to nitroglycerin, stress tests induce angina and ST-segment depression but no left ventricular contractile abnormalities at echocardiography, and sublingual nitrate administration prior exercise test execution determines earlier appearance of ECG abnormalities and/or angina.
CMVD should be identified through non-invasive tests capable of highlighting absence of contractile abnormalities and exploring the vasodilator activity of coronary microcirculation: TTDE is the first choice technique.
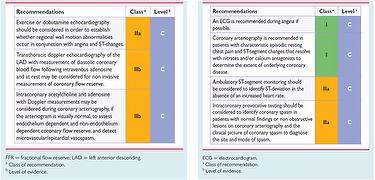
When vasodilators tests are normal or inconclusive, the response to vasoconstrictor stimuli should be assessed invasively; coronary angiogram is also necessary to obtain definite diagnosis of primary MVA, since rules out epicardial abnormalities (4). In Fig.1 ESC recommendations for diagnostic evaluation in angina without obstructive CAD are showed.
It should be remembered that a normal or near-normal angiogram does not necessarily rule out the presence of a large ‘hidden’ atherosclerotic burden, or future development of atherosclerosis stenosis. The IVDUS shows that non obstructed coronary arteries have often diffuse CAD with wall positive remodeling (wall thickens outwards without notching the lumen) (42). In patients with diffuse epicardial disease but no relevant proximal stenosis, the Fractional Flow Reserve (FFR, the ratio between distal coronary pressure and aortic pressure during maximal coronary vasodilation; a value ≤0.8 is indicative of downstream perfusion as limited to become inadequate if oxygen demand increases) (31) may be helpful to identify hemodynamic relevant coronary plaques, although no yet obstructive, and to avoid ascribing patient’s symptoms to microvascular disease (37).
Instable microvascular angina - CMVD may presents also as acute coronary syndrome, with de novo or worsening angina pattern or recurrent rest induced or mild effort induced chest pain. Microvascular angina is responsible of 10% of male and 25% of female of acute coronary syndrome and ‘normal’ coronary angiograms. Differential diagnosis should rule out transient thrombosis, coronary embolism, epicardial spasm, and two specific clinical syndromes: microvascular spasm angina and stress-related cardiomyopathy. The diagnosis requires the evidence of a cardiac ischemic origin of symptoms, suggested by new abnormalities on standard ECG which gradually reverse to normal, and sometimes by mild elevation of serum myocardionecrosis markers, the evidence of CMVD, obtained by the assessment of response to vasoconstrictor and vasodilator stimuli, and the exclusion of epicardial coronary spasm or transient coronary thrombosis. The former is achievable with provocative tests; the latter is only assumable by exclusion (4). Coronary embolism, due to atrial fibrillation or flutter, is rare, but as atrial fibrillation is often clinically unrecognized, the frequency of this mechanism of may be underestimated (38). Microvascular spasm angina (vasospastic angina) is diagnosed by intracoronary acetylcholine infusion, that reproduces angina and ST-segment changes but not epicardial spasm, and Takotsubo disease is recognizable for peculiar clinical and morphologic features. However, both these conditions are related to microvascular dysfunction, the former to diffuse coronary microvascular spasm (11), the latter to acute severe coronary microvascular constriction (39).
4 - Prognosis
Specific timelines regarding prognosis are controversial because of the heterogeneity of population included in different studies, in which presence and extent of CAD, left ventricular function impairment, and/or pathogenetic mechanism underlying angina are not taken into consideration.
Small studies carried out in patients with stable microvascular angina found rates of major cardiovascular events comparable to those of the general population (4), with the exception of re-admissions for angina (40); A more recent study records higher rate of adverse cardiovascular events in patients with SCAD and normal coronary arteries or diffuse non-obstructive stenosis compared with that in subject without CAD (41).
Approximately 20% to 30% of patients with stable MVA experience progressive worsening of symptoms (4), leading to meaningful impairment of life quality. Among subjects with angina and a normal angiogram, subgroups at high risk seem to be those with documented ischemia, mild CAD, and microvascular dysfunction. The risk of epicardial events appears during the long term (>2 years) follow-up (36).
Regarding unstable MVA, prognosis is not well known. A prospective study comparing patients with unstable and stable MVA found no major cardiac events in either group at a mean follow-up of 36 months, a similar proportion of rehospitalisation for chest pain, although 71% of patients with stable MVA complain of persistence or recurrence of angina, versus only 32% of patients with unstable MVA (4).
5 - Treatment
All patients with microvascular angina should achieve optimal coronary risk factor control. Symptomatic treatment is empirical because of the limited knowledge of its causes and the lack of conclusive therapeutic trials. Moreover, the susceptibility of symptoms to medical treatment is variable and it is necessary to experiment with different drug combinations before achieving control.
Traditional anti-ischemic drugs are the first step in medical treatment (4).
- Short-acting nitrates can be used to treat angina attacks, although they are only partially effective.
- Β-Blockers should constitute the first choice of therapy, particularly in patients with evidence of increased adrenergic activity (e.g. high heart rate at rest or during low-workload exercise), whereas they should be avoided, in vasospastic angina, as they might favour spasm by leaving α-mediated vasoconstriction unopposed by β -mediated vasodilatation.
- Calcium antagonists and long-acting nitrates are helpful in addition to β-blockers in the case of insufficient control of symptoms and can be first-line therapy in patients with a significant variable threshold of effort angina, in whom a vasospastic component is conceivable, or in definite vasospastic angina.
If anti-ischaemic drug therapy doesn't permit symptoms control, ACE inhibitors (and possibly ARBs), α-adrenergic antagonists, nicorandil, statins and estrogen replacement treatment, according to a patient risk profile, have been proposed. When these treatments are also ineffective, xanthine derivatives (aminophylline, bamiphylline) or new anti-ischaemic drugs such as ranolazine or ivabradine can be proposed (5).
Conclusion
Coronary microvascular dysfunction is a pathological mechanism of cardiac syndrome X, which causes various types of cardiac flow impairments which account for the various presentations. Microvascular dysfunction is a heterogeneous group of disorders related to several mechanisms – still largely speculative, operating alone or in combination, in the different cases. Of note, it's unknown why exposition to CVRF determines in some subject epicardial coronary arteries disease (CAD) and in others microvascular disease, or whether CMVD is the early step of CAD that progresses invariably or whether it is a distinct pathology. How to distinguish CMVD from SCAD to avoid unnecessary angiograms has also yet to be determined. Prospective studies are needed to characterise very specifically the different patients and identify subgroups at increased risk and their own prognosis.
Fig 1. Investigations recommended by ESC in patients with suspected coronary microcircular disease (A) and vasospastic angina (B) (5).


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.