Background
Prognosis allows clinicians to separate persons with heart failure (HF) into subgroups based on likely health outcomes to guide treatment. However, heart failure is a challenging condition: its heterogeneous aetiologies, varying patients' age, multiple coexisting comorbidities, varying progression of disease and uneven efficacy of therapy make this a difficult task and cause high hospital re-admission rates. Recognition of HF with preserved ejection fraction - HF-REF (HF-PEF) has also opened up the question on whether we need a separate prognostic model apart from HF-REF.
Furthermore, traditional (or conventional) risk factors do not reflect newly discovered ongoing mechanisms in HF: inflammation, oxidative stress, neurohumoral activation, myocyte stress, injury and apoptosis, excessive or inadequate proliferation of the extracellular matrix (Fig. 1). Risk stratification based on conventional predictors is often inaccurate, resulting in under-recognition of high risk patients.
Meanwhile prevalence of heart failure is high. In more economically developed countries, heart failure patients make one to two out of every hundred adults and 10 out of every hundred age 70 and above (1). The increase in the incidence and prevalence of HF-REF is due to fact that improved management and device therapy have helped many older patients to survive coronary, valvular or myocardial diseases. On the other hand, rise in obesity, hypertension and diabetes leads to development of HF-PEF (2) in the younger patient. By 2030, estimated prevalence of heart failure in the US would rise by 25%.
Costs of heart failure are expected to increase as a result of higher prevalence. Associated estimates for the 25% rise in heart failure by 2030 in the US would cause a 215% increase in annual direct medical costs.
Yet survival of heart failure patients is poor: only a 50% 5-year survival - that is the same mortality rate as that of a colorectal cancer patient for example. (3)
We argue that a more accurate assessment of prognosis, would 1) allow cardiologists to tailor a more personalised therapy, follow-up and allocation of resources - i.e according to the needs of each patient and that 2) personalised therapy would improve survival.
There is new evidence that biomarkers might be new predictors of survival in HF because they have a potential to overcome the limits of traditional predictors. Over forty biomarkers in HF have been identified. Here we present those that might be useful.
Figure 1. Categories of biomarkers in HF 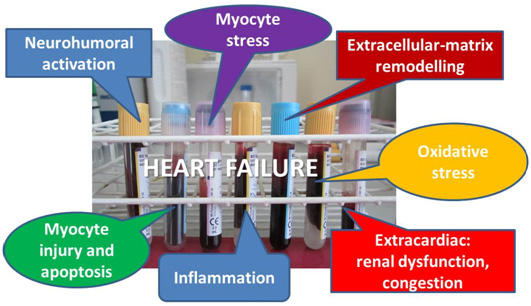
I - Description
Here presented are the biomarkers that are widely available in clinical practice: brain natriuretic peptide BNP, aminoterminal pro–B-type natriuretic peptide, NT-proBNP, high-sensitive cardiac troponin, cystatin C, tumor marker CA125 and high-sensitivity C-reactive protein (hs-CRP).
1 - Brain natriuretic peptide
We know that BNP (brain natriuretic peptide) is released from ventricular myocytes in response to pressure or volume overload the predominant stimulus of which is end-diastolic wall stress (4). We also know that both brain natriuretic peptide BNP and aminoterminal pro–B-type natriuretic peptide (NT-proBNP) release in acute HF (AHF) is stimulated by 1) myocardial stretch from volume overload 2) abnormalities of both left and right ventricular size and function 3) valvular heart disease, 4) myocardial ischemia, abnormalities in heart rhythm and 5) elevated filling pressures - including pulmonary hypertension (5).
As such, BNPs provide independent prognostic information regarding estimated risk of disease progression, hospital re-admission and mortality (6, 7, 8).
However whether admission, discharge, or change from admission to discharge levels of natriuretic peptides is the most important predictor of long-term outcomes is still being investigated.
NT-proBNP (N-Terminal pro B-type natruretic peptide)
Bayes-Genis et al. reported that among patients hospitalised with AHF, patients who experienced complications were more likely to have much smaller changes in values of NT-proBNP, (typically =15% decrease) compared to those who survived (=50% drop in NT-proBNP values from day 1 to 7). In this study, the NT-proBNP reduction percentage during admission for AHF had an area under the receiver operating characteristic curve of 0.78 (P=.002), superior to the presenting NT-proBNP concentration for this purpose.
Bettencourt et al. found that percentage change in NT-proBNP was more important in predicting HF hospitalision-free survival than absolute value at discharge.
Kociol et al. on the other hand found that discharge BNP best predicts 1-year mortality and/or rehospitalisation among older patients hospitalised with HF. Januzzi et al. comment that this may be due to the fact that a patient showing a robust reduction in BNP from 5000 pg/mL to 2500 pg/mL is still at higher risk than a patient falling from 500 pg/mL to 400 pg/mL.
Congestion is the main cause for hospitalisation and many HF patients are discharged with persistent signs and symptoms of congestion and/or a high left ventrilar (LV) filling pressure. No systematic method to assess congestion prior to discharge has been proposed by available guidelines or research studies (9). Although auscultation of rales may indicate fluid overload, the absence of rales is not a sensitive marker of the absence of congestion. Degree of change in BNP level following treatment (reduction of wet BNP) and level of BNP at discharge (dry BNP) could be used for assessment of congestion state and should be considered an important part of the pre-discharge decision making for patients hospitalised with ADHF. This use of BNP could result in reduction of re-admission rate and improvement of prognosis.
2 - Troponin
Troponin is the gold standard for the diagnosis of acute myocardial infarction (MI), but it can also occur in acute and chronic HF. According to the latest guidelines, elevated troponins are common laboratory abnormalities in heart failure. Indeed, troponin is accepted to be a predictor of adverse outcome across the spectrum of HF syndromes (10, 11).
The ADHERE investigators reported that a troponin level above the upper reference limit was associated with more severe HF, including worse LV function (although a large percentage of patients with HF-PEF had elevated troponin), more severe symptoms, more need for aggressive supportive measures and increased risk for mortality, independently of other markers of risk such as ventricular function, age, and other biomarkers such as natriuretic peptides (12). Interestingly, troponin levels were not clearly associated with HF due to ischaemic heart disease or prevalent acute myocardial infarction.
Causes that may lead to troponin elevation in HF are:
- Coronary ischaemia manifested as type I and type II MI: clinically, this is he most important cause. The supply–demand inequity in type II MI is precipitated by reduced oxygen delivery secondary to anaemia (prevalent in HF) or hypotension or increased myocardial oxygen demands due to increased transmural wall stress, dilated LV chamber size, elevated pressures in cardiac chambers, LV hyperthrophy, diastolic stiffening of the myocardium.
- Cardiomyocytes apoptosis and autophagy as a direct consequence of wall stretch, proteolysis of the cardiac contractile apparatus, direct cellular toxicity related to circulating neurohormones (such as norepinephrine), infiltrative processes, toxic exposures (e.g. alcohol or chemotherapy agents), myocarditis, as well as stress cardiomyopathy are another potential cause of high troponin in HF.
- An important extra-cardiac factor associated with measurable or elevated troponin in HF is renal failure. The exact cause of troponin release in kidney disease is not fully understood. The logical explanation can be that increased troponin levels reflect decreased clearance by the failling kidney, but it seems that the source is actually of cardiac origin.
- A large percentage of circulating troponins in chronic KD may be explained by underlying structural heart disease and/or direct toxic effects of renal failure on the myocardium, rather than caused solely by decreased renal clearance (13). Thus, elevated troponin in HF patient with renal failure should not be simply discarded as a "false positive" due to reduced clearance.
Advances in assay technology have led to more sensitive and precise high-sensitive cardiac troponin (hs-cTn) assays which have two differentiating features:
- Detection of troponin in a majority of healthy persons.
- Precise definition of what is "normal" (=the 99th percentile, which corresponds to the value of 0.014 µg/ml for troponin T).
The terms "troponin-positive" and "troponin negative" should be replaced by "detectable" and "undetectable levels" and will have to be differentiated from "elevated" levels (14) .
With the introduction of hs-cTn methods optimised for use at the troponin 99th percentile (corresponding to the upper reference limit of a healthy, normal population), it is expected that elevated hs-cTn will be more frequently detected in HF.
Xue et al. (15) and Pascual-Figal et al. (16) reported that nearly all patients with AHF had a highly sensitive troponin I or T value above the 99th percentile and found association with increased mortality.
It is likely that hs-cTn assays will improve the ability to stratify risk in HF, compared to conventional troponin tests. The value of highly sensitive troponin for prognosis is even more pronounced when it is measured serially. In patients with decompensated HF, serial increases in hs-cTn I during the course of hospitalization were associated with higher mortality than stable or decreasing TnI levels.
3 - Cystatin C
Compared to creatinin, cystatin C (Cys C) is a more sensitive marker of kidney function for detecting mild changes in glomerular filtration rate (GFR), particularly in "creatinine-blind" area (GFR range 40-90 ml/min/1.73 m2). Renal function is one of the strongest prognostic factors among patients with HF, so it is not suprising that Cys C is also recognised as an independent predictor of adverse events in HF (17,18).
In stable HF patients CysC-based measures significantly improved areas under the receiver operating characteristic curve for the prediction of major adverse cardiovascular events, especially in estimated glomerular filtration rate =≥60 mL/min per 1.73 m(2) (P<0.01).
In patients with HF-PEF, serum Cys C level on admission was also a strong and independent predictor of an unfavorable outcome. This relationship remained in patients without advanced renal dysfunction.
This draws the following questions 1) Could we attribute prognostic properties of Cys C only to the superior detection of preclinical or mild renal dysfunction? and 2) Does Cys C have prognostic value independent of renal function (19)?
Other potential contributing mechanisms that are responsible for the prognostic role of Cys C are: association between Cys C and inflammation, direct role of CysC in the vascular wall remodeling in atherosclerosis and role in remodeling of heart extracelular matrix (20).
Alterations in the heart’s extracellular matrix, composed predominantly of fibrillar collagens types I and III, can contribute importantly to structural remodelling of the myocardium that leads to ventricular systolic or diastolic dysfunction. When collagen synthesis predominates over its degradation, the resulting interstitial and perivascular accumulation of collagen will lead to fibrosis. On the contrary, when degradation of collagen predominates over its synthesis, the resulting loss of collagen will lead to the disruption of the physiological collagen scaffold. Cathepsins are released from the lysosomes to the pericellular space and degrade extracellular matrix proteins such as elastin and fibrillar collagen. Cystatins act as the endogenous inhibitors of cathepsins and inhibite degradation of extracellular matrix proteins.
Xie et al. (21) observed that in mice with HF secondary to either chronic administration of doxorubicin or left anterior descending (LAD) coronary artery occlusion, myocardial cystatin C increased, and this alteration was associated with local inhibition of cathepsin B activity and accumulation of collagen types I and III as well as fibronectin.
Diez et al. speculated that beyond its clinical usefulness to evaluate GFR, cystatin C might be useful as a mechanistic biomarker of myocardial remodelling (e.g. fibrosis) in patients with LV growth and dysfunction. This property of cystatin C may be significant in cardiorenal syndrome, in the sense that exposure of the heart to high circulating levels of cystatin C may result in myocardial remodelling, thus contributing to the high prevalence of LV hypertrophy and decreased cardiac function observed in patients with chronic kidney disease.
4 - Tumor marker CA125
Carbohydrate antigen 125 (CA125) is a tumor marker classically associated with ovarian cancer, but it may also be elevated in other cancers and benign conditions. Recently, increased serum CA125 levels have been documented in patients with HF (22).
The pathophysiological underlying mechanism for the production and secretion of CA125 in HF remains unclear, as well as its exact biological role. It has been found that CEA 125 will be released from surfaces of pleuropericardial or peritoneal mesothelial cells in response to mechanical stress such as fluid overload, and inflammatory stimuli. This explains why the elevation of CA125 in patients with chronic HF with pericardial, pleural, and peritoneal effusions and high CA125 levels were associated with higher levels of TNF-a, interleukin-6 and interleukin-1b and lower relative lymphocyte count (23).
An ensuing proposed hypothesis is that mechanical stress and inflammatory stimuli both initiate CA125 synthesis (24).
Plasma CA125 correlates with clinical, hemodynamic, and echocardiographic parameters related to the severity of the disease. High levels of plasma CA125 have been shown to be present in the majority of acutely decompensated patients, and in this setting, it has been shown to be independently related to mortality or subsequent admission for acute HF (25).
This finding suggests that CA125 might be a promising biomarker to evaluate the risk stratification of HF and monitor the process of therapy. After a few days of therapy, elevated levels of CA125 mean that patient is still in volume overload and needs more days of intra-venous diuretic therapy.
5 - High-sensitivity C-reactive protein (hs-CRP)
Inflammation is very important mechanism in the progression of HF. In the latest ESC guidelines, a CRP >10 mg/L is a common laboratory abnormality in heart failure. In a recent study of patients with AHF (26), the prognostic value of high-sensitivity C-reactive protein was investigated and it was found that mortality rate increased from the lowest to highest hs-CRP quartiles. Also after adjustment, hs-CRP was an independent predictor of 12-month mortality. When combining both hs-CRP and NT-proBNP, patients with both marker elevated had worse clinical outcomes (2.4-fold increased hazards, HR 2.382, 95% CI 1.509 to 3.761) compared to those without elevation of both markers.
II - Multimarker panel
In heart failure, the use of a single biomarker reflects only one ongoing pathophysiological pathway. The next logical step would be to cluster biomarkers in a multimarker panel in order to get a reflection of several ongoing pathological processes. Simultaneous determination of different biomarkers could optimise their application in assessment of prognosis.
Several studies have assessed multiple cardiac biomarkers simultaneously in order to gain complementary prognostic information that would be used to improve risk stratification of HF patients (27, 28, 29). A prospective study incorporated NT-pro BNP, cardiac troponin T and cystatin C, after multivariate regression analysis, found that independent and complementary prognostic information was gained. A significant gradual increased risk of mortality and/or re-admission was reported as the number of elevated biomarkers increased. The prognostic value of the multi-marker approach was found to be more powerful that the single-marker approach. Mutimarker approach could be a step toward induvidualised therapy for every patient based on the individual biomarker signal (so-called biomarker patient’s fingerprint). Depending on the dominant pathological process we would be able to create the most appropriate therapy. It would make for a very interesting topic of future studies to investigate how applied therapy could make a change in the biomarker fingerprint of patient.
Figure 2. One of the possible combinations of biomarkers that reflect different, but also common pathological pathways.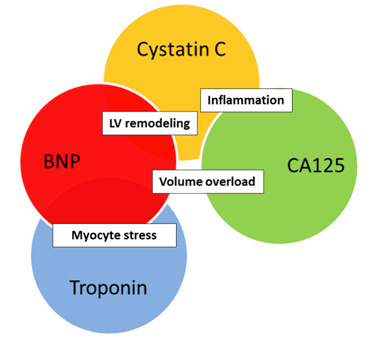
III - Costs
In the the end we come to the question: are biomarkers too expensive for routine measurement?
Although costs may differ, approximate price for tests of five biomarkers per patient is around 30 € (BNP €11, troponin €7.3, cystatin €3.6, CA125 €5.1, CRP €2.2).
In-hospital care is responsible for ∼60% of HF-related costs and median hospital cost for HF is €9,475 (1), but in many cases costs are much higher due to frequent comorbidities and need for intensive care.
If we succeed to preclude just one hospitalisation by usage of biomarkers, we will save more than €9,400 (30,31).
Conclusions
The determination of cardiac (BNP, NT6-pro BNP, hs troponin) and non-cardiac (cystatin C, CA125 and and CRP) new biomarkers can help overcome current problems with traditional predictors, especially risk stratification and recognition of newly discovered mechanisms of heart failure.
Serial measurement, the study of compared significance of various readings (admission, during treatment at discharge), of changes in measurement and better detection of lower levels offer to refine and expand the use of useful biomarkers.
- BNP as a marker of left ventricular systolic function provides independent prognostic information regarding estimated risk of disease progression, hospital re-admission and mortality.
- Hs-cTn assays will improve the ability to stratify risk in HF, compared to conventional troponin tests.
- Besides estimation of renal function, cystatin C could be used also as a cardiac marker because its reflection of extracellular matrix pathology of ventricles.
- CA125 as a marker of congestion points out degree of volume overload. CRP can evaluate progression of HF due to inflammatory etiology of HF.
In all, improved use of biomarker readings, possibly clustered in a panel may offer a cost-effective means to allow for a better prognosis and personalised treatment of heart failure patients which might in turn reduce mortality and hospital re-admission rates.


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.