Introduction
In patients with severe valvular heart disease, guideline-based surgical valve replacement or transcatheter implantation of a prosthetic heart valve is associated with improved survival and relief of symptoms. Prosthetic heart valves are designed to replicate the function of native valves by maintaining unidirectional blood flow and can be separated into two broad categories, mechanical and bioprosthetic (also called tissue) valves, each with different advantages and disadvantages.
This mini-series is divided into 4 parts:
Part 1 – Prosthetic valves: selection
Part 2 – Prosthetic valves: antithrombotic therapy
Part 3 – Prosthetic valves: imaging
Part 4 – Prosthetic valves: complications and dysfunction, pregnancy
Cardiac imaging modalities
Echocardiography
Transthoracic echocardiography (TTE) is the primary non-invasive imaging modality for evaluation of mechanical as well as bioprosthetic valves (BPVs). It allows both morphologic and haemodynamic assessment according to the current guidelines [1,2]. The integrative approach to assess prosthetic function includes 2-dimensional, colour Doppler, pulsed-wave and continuous-wave Doppler information and can be performed in a serial fashion.
While a comprehensive transthoracic Doppler interrogation with determination of functionality metrics is a validated and reproducible technique for detection of prosthetic valve (PV) stenosis, transoesophageal echocardiography (TEE) as a complementary modality is particularly helpful for assessment of PV structure and regurgitation, especially in mechanical mitral and tricuspid prostheses. However, a limitation of both transthoracic and transoesophageal echocardiographic imaging is acoustic shadowing and reverberations created by valve components in mechanical but also BPVs.
Normal BPV leaflets are thin, and appropriate leaflet morphology and movement can be assessed from multiple echocardiographic views. The preprocessing zoom mode of real-time images with increased frame rates and better image resolution improves visualisation of PV structure and leaflet motion. The struts and frame on which the bioprosthetic leaflets are attached are strong echo-reflectors and may lead to acoustic shadowing or reverberations obscuring the view on the leaflets, particularly in the left parasternal view. However, this phenomenon is of lesser magnitude than in mechanical heart valves (MHV).
A comprehensive TTE after valve implantation should be performed before hospital discharge or at the first visit, 2 to 6 weeks after hospital discharge, when the chest wound has healed, ventricular function has recovered, and anaemia with its attendant hyperdynamic state has improved. This index echocardiographic examination provides an assessment of the procedural result and serves as a baseline for follow-up comparisons [1-4].
Routine follow-up examinations in patients with normally functioning BPVs are recommended annually thereafter [4], beginning 10 years after implantation [3], because the incidence of clinically important structural valve deterioration increases markedly more than 10 years after surgery. In patients with mechanical valve prostheses, routine follow-up echocardiography is not needed if the postoperative baseline study is normal and no clinical change is apparent. However, many of these patients require TTE studies for other indications, such as for the assessment of right and left ventricular function, other cardiac or valve disease, or aortic dilatation at the time of surgery.
Studies should also be performed when new cardiac symptoms occur or new murmurs are detected on auscultation. Durability data for transcatheter valves are less robust than for surgical valves. Transcatheter aortic valve implantation (TAVI)-based protocols typically require routine TTE soon after implantation to establish baseline valvular function, and subsequently at 1 to 3 months and 1 year, in part because of reporting requirements. With lack of long-term data, routine annual TTE studies are reasonable as experience continues to accumulate [1,3,5].
The principles of Doppler echocardiography used in the evaluation of native valve disease also apply to PVs with a few distinctive characteristics. Pressure gradients are calculated from Doppler velocity information using the Bernoulli equation. The mean gradient is a standard measure of PV haemodynamic performance. However, transprosthetic Doppler velocities and gradients are both flow-dependent and dependent upon valve type, size and location. Effective orifice area (EOA) calculation is based on the continuity equation concept and for prosthetic aortic valves (PAV) includes three measured parameters: the left ventricular outflow tract diameter (LVOTD), the LVOT pulsed-wave Doppler velocity-time integral (VTI), and the transprosthetic continuous-wave Doppler VTI (the respective peak velocities may be used instead of the VTIs):
EOAPAV = LVOTD² × π/4 × VTILVOT / VTIPAV
Among these parameters, the LVOTD measured at the inflow aspect of the PV from its external border to the external border of the sewing ring or stent is the most critical one because, first, it is technically challenging to measure, and second, it is squared in the continuity equation. Importantly, the PV label size cannot be used as a surrogate for the LVOTD because it does not reflect the true external diameter and shows great variability among different manufacturers and valve designs. Moreover, the pulsed-wave Doppler sample volume should be precisely positioned immediately proximal to the sewing ring or apical portion of the stent of the PV to avoid the flow acceleration within the stent or at the level of the leaflets, which would result in an overestimation of the calculated EOA that is based on the ratio of pre-prosthetic to transvalvular flow velocities. If stroke volume calculation at the pre-prosthetic site is not possible or accurate for technical or anatomical reasons, the Doppler-derived stroke volume can be replaced by the 2D-derived stroke volume according to the biplane method of discs (modified Simpson’s rule) in the absence of significant mitral regurgitation (MR) [6].
The Doppler velocity index (DVI) is a dimensionless parameter, which is calculated by dividing the LVOT (pre-prosthetic) VTI by the transprosthetic VTI without the need to measure the LVOTD, thus eliminating a potential source of error. This approach is particularly helpful when the cross-sectional area of the LVOT cannot be obtained for technical or anatomical reasons. The DVI can also be approximated as the ratio of the respective peak velocities. For a normally functioning aortic prosthesis, the DVI is typically 0.30-0.35. This number has no numerical equality because no PV in current use has an EOA as large as that of the respective native valve. Hence PVs always have an inherent obstructive element and patients with normally functioning PVs have mild to moderate stenosis. This circumstance provides an argument for implanting an aortic PV as large as possible and, if necessary, extension of the surgical procedure to include an aortic root enlargement as well.
The EOA measured by Doppler is generally smaller than the anatomic (or geometric) valve area measured by 2D planimetry or the EOA measured by cardiac catheterisation using the Gorlin formula. This discordance is related to the flow contraction and pressure recovery phenomena. Blood flow passing through a prosthetic aortic valve reaches the maximum velocity downstream from the PV and distal to the actual smallest anatomic area at the level of the vena contracta (VC). As blood flow decelerates, a proportion of the kinetic energy (dynamic pressure) is not dissipated as heat but recovered as potential energy corresponding to an increase in static pressure in the aorta, so-called pressure recovery.
Doppler-based methods always detect peak flow velocities that occur at the narrowest portion of the VC. The EOA calculated by the continuity equation corresponds to the minimal cross-sectional area of the transprosthetic flow jet and may therefore underestimate the true anatomic orifice area theoretically available for the bloodstream to pass through the PV.
While several studies in native valve disease have evaluated the method of measuring the anatomic or geometric valve area by direct planimetry as an alternative to Doppler estimation, there are only limited data for prosthetic valves by means of 2D or 3D echocardiography [7] and allied techniques [1]. This approach is currently not used routinely for clinical purposes.
For prosthetic mitral valves (PMV), the DVI is calculated as VTIPMV / VTILVOT (normal <2.2 for mechanical mitral valves) and the EOA estimated as stroke volume (SV) through the prosthesis divided by the transprosthetic VTI (measured with continuous-wave Doppler to ensure that maximal velocities are recorded):
EOAPMV = SV / VTIPMV
The stroke volume through the mitral valve is often equated with the LV forward stroke volume through the LVOT in the absence of significant aortic regurgitation (AR) and MR or a confounding intracardiac shunt between both ventricles. In contrast to Doppler velocities and gradients, EOA and DVI provide a less flow-dependent evaluation of prosthetic valve haemodynamics. There is significant variability in quantitative parameters of normally functioning PVs because of different types and sizes. Individual valve parameters of velocities, gradients, and EOAs for various surgical PV types and sizes in the aortic and mitral position are compiled in the respective guidelines [1,2] and more recently also for transcatheter aortic valve systems [8]. Importantly, the reference values for aortic PVs reported there already incorporate the pressure recovery phenomenon.
Detection and grading of prosthesis regurgitation involve several Doppler echocardiographic parameters and follow the same principles and methods used for quantitation of native valvular regurgitation [9-11]. However, they are more challenging, particularly in mechanical prostheses because of shielding and reverberations and the presence of multiple and eccentric jets. In BPVs, trivial to mild central valvular regurgitation on colour Doppler may be a normal finding due to minimal leaflet retraction provoked during implantation (Figure 1).
Figure 1. Colour flow Doppler images of physiological regurgitation in aortic prosthetic valves by transthoracic echocardiography (top) and mitral prosthetic valves by 2D and 3D transoesophageal echocardiography (bottom).
Trivial to mild valvular aortic regurgitation in a normally functioning BPV with the jet originating at the central coaptation point of all 3 leaflets (top left). Minor built-in regurgitation with 4 small and symmetrical jets in a mechanical bileaflet aortic valve (top right). A mechanical bileaflet mitral valve in closed position showing several regurgitant “washing jets” in systole in 2D imaging (bottom left). The same image from the atrial side in a 3D perspective obtained with multiple-beat acquisition (bottom right).
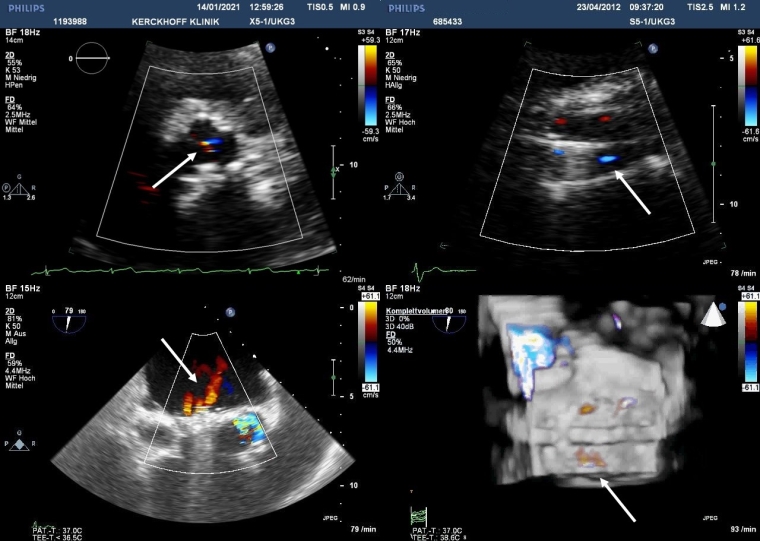
Minor, physiologic regurgitation is also inherent to all mechanical valves. First, a small amount of closing volume triggered by the backward motion of the occluder may be visible. Second, all mechanical valves with the exception of caged ball valves (no longer implanted) have trivial to mild transvalvular regurgitation at the edges of the leaflets. This “built-in” regurgitation theoretically prevents blood stasis and thrombus formation using a washing effect (Figure 1). This normal leakage backflow consists of one (Medtronic-Hall valve) or more (bileaflet valves) narrow and symmetrical jets inside the sewing ring and should be distinguished from aberrant paravalvular flow external to the sewing ring. Although periprosthetic regurgitation is abnormal, small single or multiple jets are not uncommon because annular calcification or fibrotic scar may lead to incomplete apposition of the sewing ring against the annulus. Technical factors during the surgical procedure and suture disruption from mechanical causes at a later point in time may also play a role. Finally, new paravalvular aberrant regurgitation may also be due to infective endocarditis.
The language used to describe valve structure and position as well as the site of regurgitation should preferably incorporate anatomic landmarks (e.g., anterior/posterior, medial/lateral) or adjacent structures in relation to the annulus instead of a description according to a clock face, in order to improve communication and facilitate comparison with follow-up examinations. The origin of prosthetic AR (central vs paravalvular) is best imaged in the parasternal short-axis view. Care should be taken to image the entire circumference of the sewing ring by obtaining multiple imaging planes at and below the level of the sewing ring (Figure 2).
For paraprosthetic aortic jets, the proportion of the circumference of the sewing ring occupied by the neck of the jet gives an estimate of severity (<10% = mild; 10–29% = moderate; ≥30% = severe) [12,13]. This semi-quantitative method, however, does not account for the radial thickness of the leak and has not been validated for multiple jets where the respective values of width are not additive. There is also a risk of overestimating the regurgitation severity, especially in eccentric jets below the valve, compared with quantitative methods.
Paravalvular regurgitation is one of the most frequent periprocedural complications of TAVI and frequently arises from multiple sites, making assessment by colour Doppler echocardiography even more difficult. The severity and extent depend on anatomic and morphologic features of the aortic annulus and affect both short- and long-term prognosis [14]. Specifically, calcium at the commissures is a risk factor for post-procedural AR originating from the corresponding commissure. For the same reason, bicuspid aortic valves with heavy and asymmetric calcification have been demonstrated to predispose to leak development. Preliminary data indicate that newer-generation valves show significantly reduced perivalvular regurgitation. Currently, it is unknown whether this procedural benefit translates into improved long-term clinical outcome.
The assessment of severity of paravalvular AR is challenging and requires a multi-window, and multi-parameter integrative approach, including the circumferential extent of the jet(s) described above but also the jet width at its origin, the VC width if adequately visualised, the pressure half time, and the flow reversal in the descending aorta. Quantification of regurgitation by the proximal isovelocity surface area (PISA) method is limited in PVs as the PISA shells are rarely hemispheric and often truncated by adjacent walls except in BVs with central valvular regurgitation. The VC is the narrowest portion of the regurgitant flow that occurs at the prosthesis or immediately downstream and corresponds to the effective regurgitant orifice area (EROA). Due to the shadowing caused by the prosthetic material, the VC width may be difficult to assess. There are limited data on the application and validation of this parameter in the context of PHVs, particularly in case of multiple jets or irregular orifice shape. Hence, the best way to quantify the degree of PV regurgitation is the volumetric approach.
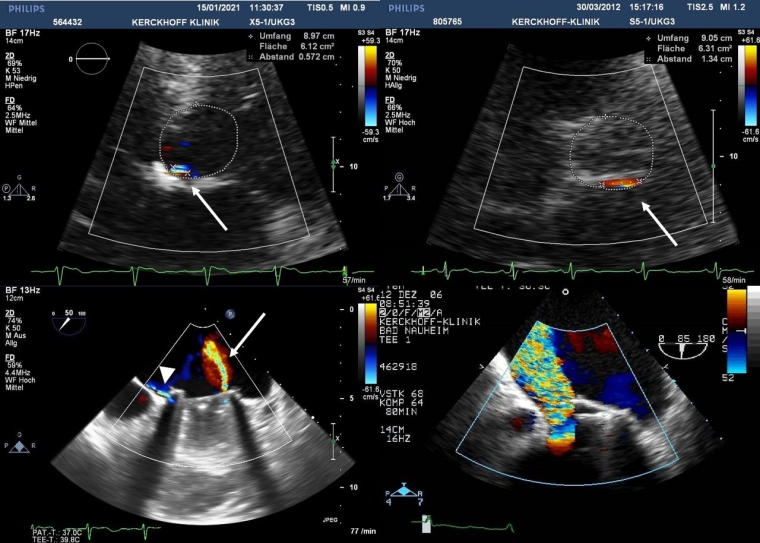
An integrative approach with a unifying 5-class grading scheme has been proposed by the Valve Academic Research Consortium (VARC) for paravalvular leaks in surgical valves [13]. Indirect signs from various Doppler parameters can suggest the presence of significant regurgitation. For example, a high transprosthetic Doppler gradient in conjunction with a normal EOA may be a clue to significant prosthetic regurgitation. In addition, the severity and chronicity of PV regurgitation invariably lead to cardiac remodelling, which represents a supportive but not specific indicator for PV regurgitation severity. TTE is typically inadequate for evaluation of prosthetic MR and needs to be supplemented by TEE, especially for differentiation of valvular from periprosthetic regurgitation (Figure 2).
Figure 2. Colour flow Doppler images of paraprosthetic regurgitation in bioprosthetic aortic valves by transthoracic echocardiography (top) and mechanical mitral prosthetic valves by transoesophageal echocardiography (bottom). The neck of the regurgitant jet located posteriorly in a short-axis view comprises 7% of the total ring circumference equivalent to mild (top left) and 14% equivalent to moderate (top right) paraprosthetic regurgitation, respectively. Physiologic straight regurgitant jet inside the sewing ring (arrow) and a very eccentric paraprosthetic jet outside the sewing ring (arrowhead) in a single tilting disc mitral prosthesis (bottom left). Severe paravalvular leak due to mechanical mitral valve prosthesis dehiscence (bottom right).
Cardiac computed tomography
Multidetector computed tomography (MDCT) offers excellent spatial resolution and rapid acquisition times in conjunction with isotropic imaging, where multiplanar reconstruction images can be created in any imaginary cross-sectional plane with the same spatial resolution as the original ECG-synchronised acquisition. MDCT is generally not performed for routine evaluation, but can provide incremental information on PV structure and periprosthetic pathology (e.g., abscess or aneurysm) and is suitable for distinguishing between thrombus and pannus. If data are acquired throughout the cardiac cycle, then images can be reconstructed representing consecutive cardiac phases, which subsequently allow dynamic assessment of leaflet mobility in MHVs. CT imaging of the cusps of biological valves can help to detect reduced leaflet motion and morphologic abnormalities such as leaflet thickening, calcification, or thrombus [1].
Functional imaging also permits determination of right and left ventricular stroke volumes which can provide an estimate of PV regurgitation in the absence of other concomitant valve regurgitation. However, this comes at the cost of increased radiation exposure and administration of iodinated contrast agents and may only be indicated in the context of non-diagnostic echocardiography and contraindications to CMR. Similar to coronary CT protocols, pharmacological heart rate modulation may be required. In the context of severe prosthesis dysfunction, aggressive rate control with beta-blockade may be contraindicated.
Despite excellent spatial and temporal resolution, the quality of CT images is affected by MHV-related artefacts to varying degrees according to the metal components. Blooming artefacts from metal struts can also occur in stented BPVs. Moreover, MDCT is further limited by its inability to assess flow and pressure gradients.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is a non-ionising imaging technique utilised increasingly for non-invasive assessment of cardiac structure and function. Cardiac or extracardiac MRI examinations are considered safe in patients with PHVs (mechanical, bioprosthetic and transcatheter) any time after implantation at field strengths of up to 1.5 T and for most prosthetic valves at a higher field strength of 3 T without any risk of valve dehiscence and only minor MRI-related heating [15]. This is also true for patients who have sternal wires. Relevant MRI safety information for any cardiac device is also available in online resources (e.g., www.mrisafety.com).
No established guidelines exist on how to use CMR for assessment of PV structure and function. The role of MRI in the morphologic diagnosis of structural valve degeneration remains to be determined. However, CMR imaging allows quantification of prosthetic regurgitant volume and fraction by direct and indirect methods. The direct method employs through-plane phase-contrast CMR to quantify antegrade and retrograde blood flow at a given location (e.g., semilunar valves). Alternatively, indirect quantification is possible by comparison of right and left ventricular stroke volumes or a combination of both methods (such as the difference between the LV stroke volume and aortic flow to quantify MR). All these CMR approaches may be limited by the occurrence of image artefacts from metallic components of the PVs. Moreover, in contrast to echocardiography, there is a paucity of data on specific CMR thresholds that define severe regurgitation. In summary, while 2D and Doppler echocardiography remains the first-line imaging modality for serial assessment of PV function (reflecting the global burden of valvular disease), CMR may be useful as a complementary tool.



 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.