Paravalvular or paraprosthetic leak (PVL) is a complication associated with the implantation of a prosthetic heart valve whether traditional (surgical) or a transcatheter (TAVI) approach. PVLs have so far most commonly been observed in patients surgically implanted with either bioprosthetic or mechanical valves.
Paravalvular or paraprosthetic leak refers to blood flowing through a channel between the structure of the implanted valve and cardiac tissue as a result of a lack of appropriate sealing. The majority of PVL are crescent, oval or roundish-shaped and their track can be parallel, perpendicular or serpiginous. PVL is most commonly observed with mechanical valves followed by bioprosthetic valves. Incidence of PVL, including small non-significant jets, is estimated to be as high as 20%. PVL is also more common with mitral (up to 20%) than aortic prostetic valves. Of note however, this apparently high incidence was reported in studies using transesophageal echocardiography (TEE) which is able to also detect small, non-significant jets. The presence of clinically overt PVLs that warrant repair has occured in 1-5% of patients with prosthetic valves [1-4].
Etiology and mechanism of aberrant paraprosthetic flow
PVL is most commonly related to disruption of sewing ring sutures precipitated by infectious endocarditis accompanied by an abscess formation or significant calcification and fibrotic scar of the annulus. Technical factors during the surgical procedure may also play a role. They may result in incomplete apposition of the valvular structure against the annulus leading to formation of single or multiple jets located externally to the sewing ring. In most cases, paravalvular flow is present immediately after valve implantation but is mild and causes no symptoms [1-4].
A - Presentation
Signs and symptoms of significant PVL are heart failure (HF), hemolytic anemia and infectious endocarditis. Hemolysis can be severe enough to precipitate symptoms of anemia or they can also be mild. In latter cases, asymptomatic hemolysis is diagnosed based on reduced hemoglobin levels, elevated lactate dehydrogenase activity (LDH), changed reticulocyte counts and bilirubin levels, as well as reduced haptoglobin concentrations [5] [6].
Although natriuretic peptides measurements are not commonly conducted in studies investigating PLVs, our registry showed that the majority of patients with significant PVL present with elevated levels of N-terminal pro-B-type natriuretic peptides (NT-proBNP). Importantly, percutaneous repair of PVLs lead to a marked reduction in NT-proBNP levels (unpublished observation).
B - Diagnosis
Once the signs and symptoms suggestive of PVL have developed, the most important diagnostic modality is echocardiography. Sucessful imaging of the PVL can be challenging. Transthoracic echocardiography (TTE) often cannot differentiate the PVL from prosthetic regurgitation which is a result of degenerative changes of bioprosthetic valve or pannus ingrowth in the mechanical valve. In such a situation, TEE is crucial and often requires multiple projections. The challenges of echocardiography in thes situations are related to the presence of thrombi, pericardial fluid, artifacts and acoustic shadows caused by the structure of the prosthetic valves [4].
Echocardiographic evaluation of PVL should provide the following information:
- Shape and orientation of the jet
- Number of jets
- Maximum velocity
- Presence of the distal flow reversal
- Pulmonary pressures
TEE is also more useful than TTE in evaluating the shape of paravalvular defect, in assessing excentric jets and differentiating between single and multiple jets. Major progress in TEE in the area of PVL imaging has been 3-dimensional reconstruction which is also very helpful in guiding percutaneous repair. When performing echocardiographic imaging, the operator should be informed on the type and size of the prosthetic valve, the preferred timing for and technique of implantation including the technique for sewing sutures and the immediate postoperative as well as follow-up results of echocardiography [7-8].
Several findings on TTE and TEE are suggestive of significant aortic PVL, such as: short pressure half time (PHT), “dense” regurgitation jet in CW Doppler imaging, descending aorta diastolic flow reversal, regurgitant fraction >50%, increased systolic transvalvular gradient despite normally functioning prosthesis and lack of LVEDV reduction following aortic valve replacement (AVR) procedure in patients operated on for significant aortic regurgitation.
Indirect markers of significant PVL on prosthetic mechanical mitral valve are: diastolic increase of maximum velocity > 1,9m/s and mean gradient > 5mmHg, velocity-time integral (VTI) across the valve prosthesis to VTI in left ventricular outflow tract (LVOT) ratio > 2,5, maximum velocity of tricuspid regurgitation > 3m/s [7, 8].
Measurement of the ratio of total sewing ring circumference to the length of sutures dehiscence offers a semiquantitative assessment of the size of PVL (<10% - mild, 10 – 20% - moderate, >20% - severe, > 40% - instability of the prosthetic valve).
Traditional echocardiography (TTE, TEE) can be supported by 3D reconstruction, intracardiac echocardiography, as well as by multislice computed tomography (MSCT), ventriculo- and aortography, whereas the use of magnetic resonance imaging is limited. Rarely is direct measurement of pressures during cardiac catheterisation carried out.
MSCT, 3D echocardiography and intravascular ultrasound (IVUS) can be used to evaluate size, shape, track and relationship of the paravalvular canal to the sewing ring and prosthesis structures. Such information is crucial if the transcatheter repair is to be attempted.
C - Treatment
Significant PVLs can be treated either surgically or using the transcatheter deployment of the occluder devices (plugs). Reoperation is first-choice procedure when PVL coexists with significant dysfunction or mechanical instability of the prosthetic valve, need for by-pass surgery (CABG), and infectious endocarditis. Registries showed that surgery reduced mortality from 26 to 12% in comparison to conservative management [9]. In many patients however the operative risk is prohibitive and long term follow-up is often complicated by recurrent leak and increased mortality [1-3].
Recently, transcatheter closure of PVL has emerged as a new treatment strategy that can be offered to patients with isolated PVL or to those with a very high risk of repeat surgery [10].
The transcatheter approach involves deployment of occlude devices or coils and adopting either a percutaneous or a transapical approach.
Percutaneous approach:
- Access through the femoral vein and transseptal puncture (mainly for treatment of mitral valve PVL)
- Retrograde approach through femoral artery (mainly for treatment of aortic PVL)
The transapical approach involves puncture of the apex either using small thoracotomy or percutaneous access (direct puncture).
Despite the differences in access sites the principles of the transcatheter closure of PVL remain the same. After passage of the catheter in the proximity of the PVL canal, the guidewire is passed across the canal and the guide is advanced inside. Using TEE guidance, the dedicated occluder (plug) is deployed and the results checked. For this procedure either purpose-specific plugs (Vascular Plug III) or other types of occluders used commonly for closure of ventricular septal defects or patent ductus arteriosus can be used. Use of coils for narrow PVL canal closure was also reported.
Aortic prosthetic valve PVL is usually repaired using a retrograde approach through the femoral artery and the mitral PVL can be closed either retrograde after passage through the aortic valve or antegrade using atrial septal puncture. Several technical issues can make the procedure challenging. The primary problem is the localization and track of the canal which can be very difficult to engage and cross with the guidewire. Also the maneuverability of the guide catheter in often enlarged left atrium and the passage of the occluder device through narrow and serpiginous canal between calcified annulus and sewing ring is challenging. The choice of the occluder device can determine procedural success. In most round-shaped PVL the PDA or VSD-dedicated occluders can be used, whereas crescent-shaped PVL elipsoid AVP III plugs might be more appropriate [11]. The size of the occlude is determined by the canal’s diameter as well as the proximity of the prosthetic valve, especially when it comes to mechanical prosthesis. Appropriate sizing will prevent the interference of the occluder disk with the leaflets.
Procedure of the PVL closure can be complicated by cardiac tamponade, migration and embolisation of the device, dysfunction of the prosthetic valve caused by interference with occlude, formation of thrombus and access site bleeding. In addition, the residual leak may be associated with increased intravascular hemolysis [12,13].
So far the data on the efficiency of transcatheter PVL closure is sparse. Most of the published series involve small numbers of patients. In most publications the procedural success rate was between 40 and 90% [5].
The transapical approach can be an alternative in cases of a failed attempt using th percutaneous route or significant difficulties in the passage through the leak canal. Transapical procedure is carried out in the hybrid operation room using the fluoroscopic guidance as well as TEE. Procedure is performed under general anesthesia by lateral minithoracotomy or direct percutaneous puncture of the apex.
Such an approach is especially useful in the setting of mitral PVL because it does not require the transseptal puncture, challenging navigation in the left atrium and provides direct access to the valve. The experience with transapical PVL closure is limited even in the high volume structural heart disease centers, however in most cases the procedural success rate is high [11].
Fig 1. Aortic valve mechanical prosthesis PVL closed with AVP III
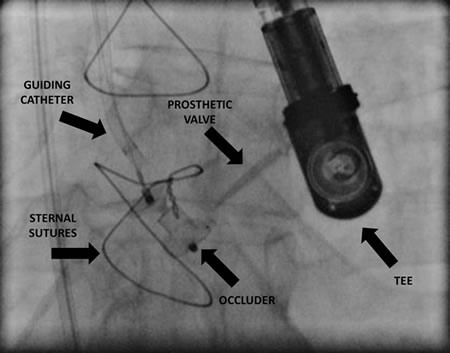
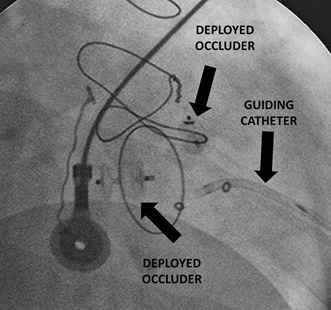
Fig 2. A mitral valve PVL closed with two AVP II occluders using the transapical approach


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.