Background
Takotsubo cardiomiopathy (TC) was first described in Japan approximately 20 years ago (Satoh and coworkers, 1991). Its name refers to a contraption used for catching octopuses and suggests the aspect assumed by the ventricle during the systole due to the typical regional wall motion abnormalities that occur after onset. Since its first description, this condition has been increasingly recognized: over 813 articles have been reported on this condition and are available on Medline/PubMed. However, certain reports identifying presumed “new” entities (such as apical ballooning syndrome, ampulla cardiomiopathy, broken heart syndrome) characterised by nearly the same clinical features of TC have generated uncertainties concerning the nomenclature and debate is ongoin. (1)
Takotsubo seems to be an uncommon cardiomopathy. Precise incidence is unknown, however up to 0.7 - 2.5% of all patients presenting with an initial clinically suspected acute coronary syndrome (ACS) may have a TC and the overall incidence is likely to be underestimated. It occurs mainly in postmenopausal women, with a mean age of 68 years and a higher prevalence in the 7th/8th decades of life (2).
I - Pathophysiology
The causes of this syndrome are still unclear. Several ethiopathogenetic mechanisms have been advocated by authors, but further studies are warranted:
- Multivessel vasospasm of epicardic coronary arteries as in Prinzmetal angina. (3)
- Acute microvascular spasm. (4)
- Excessive sympathetic stimulation. Markedly elevated serum concentrations of catecholamine may induce myocardial metabolic impairment and stunning. (5)
- Neurogenic stunned myocardium caused by an acute cardiac autonomic dysfunction. (6)
- Coronary artery disease with aborted myocardial infarction for spontaneous thrombus lysis: transient coronary occlusion by a fast-dissolving clot and spontaneous reperfusion. (7)
- Other
Irrespective of its underlying aetiology, it has been hypothesised that TC could be characterised by a common pathophysiological pattern involving acute and reversible coronary microvascular dysfunction. The occurrence of the syndrome in postmenopausal women may support the hypothesis of stress-mediated vasoconstriction enhanced by estrogen depletion. In a recently published study, a perfusion defect was evident at myocardial contrast echocardiography (MCE) within the dysfunctional myocardial area. As opposed to a group of anterior STEMI control patients, the extent of perfusion defect was transiently reduced by the infusion of adenosine (that vasodilates constricted microvessels) and entirely resolved within a 1-month follow-up. The finding that microvascular defect promptly returned at baseline soon after adenosine challenge reveals the functional (vs anatomical) nature of microvascular dysfunction. Interestingly, the study has also reported a transient contractile recovery that paralleled the improvement of perfusion, within 90 seconds from adenosine administration, suggesting new therapeutic targets. It is possible to assume that the vasodilator agent, enhancing the blood flow in the viable myocardium, could elicit the contractile reserve and/or produce an increase in myocardial thickness resembling a regional improvement in contractile function. (8)
II - Clinical Presentation
TC resembles acute myocardial infarction (AMI). Often, the event is triggered by intense physical and emotional stress or by medical procedures and non-cardiac surgery. Interestingly, occurrence of TC following some quite unusual triggering conditions such as successful resuscitation of out-of-hospital cardiac arrest and acute diarrhoea have been recently reported. (9, 10) Certain cases present no detectable evidence of stress.
Chest-pain with electrocardiographic changes is the most typical early presentation, however TC patients have presented dyspnoea or heart failure. The release of myocardial necrosismarkers (troponin or creatine-kinase MB) would normally be a minimum for diagnosing TC along with a coronaric angiography that does not reveal any significant lesions.
Commonly, patients with TC present either no angiographically detectable coronary disease or non-obstructive coronary disease (epicardial stenosis greater than 50% of the luminal coronary artery diameter). Ventricular dysfunction is associated with transient alterations of the contraction mechanism usually extended beyond a single vessel territory and demonstrable with transthoracic echocardiography.
Although prognosis is generally favourable with a prompt and spontaneous recovery, TC is not always a benign disease since several complications can also occur. For example it may be associated with a formation of a thrombus and represents an exceptional risk for embolic events, such as brain infarctions. (11) The thrombus usually disappears within a few weeks following the beginning of anticoagulation therapy. A case of acute ischemia of the right superior arm has been recently reported as an anomalous “early” clinical presentation of TC, which required surgical removal of an intraventricular thrombus. (12)
A - Electrocardiography
Close to all types of ECG abnormalities are possible on admission electrocardiograms: ST segment elevation in the precordial leads, diffuse T-wave inversion, pathological Q-waves, QT prolongation and conduction abnormalities (new left or right bundle branch block). (13) Recent studies have aimed at assessing the value of ECG in discriminating TC from the results of acute coronary occlusion (especially from the anterior AMI). (14) This differential diagnosis, although important for selecting the appropriate treatment strategy, probably cannot be based on ECG changes alone.
B - Imaging
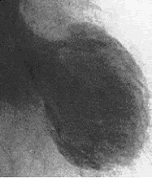
The hallmark morphological feature of TC is the extensive hypo-akinesia of apex the and lateral ventricular segments in conjunction with compensatory hyper-kinesis of the base. But, above all, the complete recovery of ventricular function witin a few days or weeks after admission is typical. In this regard the echocardiogram in the acute phase of TC has a crucial role, since it is usually suggestive of the condition, demonstrating the ventricle with a ballooning of the apex resembling the octopus trap configuration along with a reduced ejection fraction.
Given its non-invasiveness and minimal associated patient discomfort, 2-dimensional transthoracic echocardiography is an important tool not only for helping with diagnosis but also for follow-up purposes. (15) Three-dimensional echocardiography using volume slopes, however, has been shown to reveal the relevant changes more clearly in the systolic wall motion. (16) Other helpful techniques are gated myocardial perfusion imaging, computed tomography and magnetic resonance. TC patients will usually also present a typical left ventriculogram, demonstrating the takotsubo-shaped left ventricle. The left ventricle is predominantly affected, even though variants with biventricular involvement have been described and were associated with a more severe left ventricle dysfunction as a result. The first documented case of “isolated” right ventricular stress cardiomiopathy has been reported recently. (17)
Fig. 1 : Takotsubo cardiomiopathy as seen by left ventriculography
III - Diagnosis
Not one clinical characteristic of this syndrome alone is considered specific, per se. As a consequence diagnosis of TC remains ultimately confined to awareness and sound clinical judgment.
Although they lack general acceptance, four diagnostic criteria have nevertheless been suggested :
- Newly diagnosed ECG abnormalities
- Transient apical dyskinesia or akinesia, detected by Ecocardiography colordoppler, beyond a single coronary artery distribution
- Nonobstructive coronary artery disease (stenosis < 50%) at angiography
- Absence of: myocarditis, pheochromocytome, head trauma and intracranial haemorrhage, hypertrophic cardiomiopathy.
In diagnosing TC, the catheterization laboratory holds an important role: the almost unique association of normal coronary arteries and extensive apical akinesia may be demonstrated in the acute phase of the disease.
According to recent results, simple parameters derived from LV angiograms enable the clinician to differentiate TC from ST-segment elevation myocardial infarction (STEMI) with a very high sensitivity and specificity. (18) However, since TC does not derive any benefits from angiography (other then ruling out STEMI), it is obvious that any diagnostic criteria that may adequately support diagnosis of TC before coronary intervention would save further unwarranted procedures.
IV - Treatment
Although poor scientific evidence regarding best treatment strategy for this transient cardiomiopathy is currently availablen emerging data supports the role of beta blockade. (19) Nevertheless in a recent retrospective analysis of 40 TC cases (F:M = 36:4; mean age 69, 4 ± 11, 41 years) from an international multicentric study, we failed to detect significant advantages derived from any one of the seven approaches to pharmacological treatments that were used during the acute phase. (20, 21)
In all, management of Takotsubo cardiomiopathy is primarily empirical and needs to be individualised for each patient. Nevertheless, various reports confirm the need for anticoagulation therapy in certain TC patients. (11) Furthermore, the role of vasodilators such as calcium channel blockers, endothelin antagonists and adenosine is likely to become well established in future trials. (8)



 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.