MRI provides a new, non invasive, non ionising approach for the assessment of myocardial perfusion both at rest and during pharmacological stress. The detection of inducible perfusion deficits by MRI can be proposed as a routine clinical test in the evaluation of coronary artery disease and offers the advantage of superior spatial resolution.
In coronary artery disease, the interaction for a revascularisation procedure such as percutaneous angioplasty or coronary artery bypass surgery is often based on the documentation of inducible ischemia by non invasive tests aimed at the evaluation of either perfusion reserve by Nuclear Medicine or contractility reserve by stress-Echo.
However, Nuclear Medicine techniques have limited spatial resolution and involve the use of ionising radiation while Echocardiography is largely operator dependent.
Magnetic Resonance Imaging (MRI), combining real time ultra-fast imaging acquisition with the bolus injection of a contrast agent, is emerging as an alternative non-invasive, non ionising technique for studying myocardial perfusion at rest and during pharmacological stress (1).
The most commonly used contrast agents are chelates of gadolinium, which shorten T1 relaxation time leading to an increase in Signal Intensity (SI) on T1-weighted MR images. Although during the first pass more than 30% of the contrast agent enters the interstitium, the concentration of contrast agent in the myocardium during the wash-in phase is primarily determined by myocardial blood flow.
After the intravenous bolus administration of a contrast agent, normal myocardium will show increased signal intensity, followed by contrast washout. The contrast agent dose determination requires a compromise between optimality of signal-to-noise ratio and avoidance of the non-linear range of contrast-signal relationship, which can occur at higher concentrations of the gadolinium-based agents. Accurate characterisation of regional myocardial perfusion necessitates repetitive acquisition of images over the entire heart with high temporal resolution to capture the first pass of contrast agent through the myocardium. Usually three to seven short axis views of the left ventricle are acquired covering 16 out of 17 possible myocardial segments. As the most distal part of the apical region is missed, this does not reduce the diagnostic efficacy of this approach. Image acquisition is repeated for several consecutive heart beats to follow the first pass of the contrast agent. Evaluation of perfusion reserve implies that a second set of images are acquired after administration of a pharmacologic agent such as dipyridamole or adenosine.
Depending on the degree of injury, the ischemic zone appears with a delayed and lower signal enhancement when compared to a normally perfused myocardium. Figure 1 shows images in a basal condition with normal perfusion, a homogeneous signal from the whole myocardium (left panel) and during adenosine stress with subendocardial ischemia in the inferior and infero-septal segments visualised as hypo-intense signal (right panel).
Figure 1
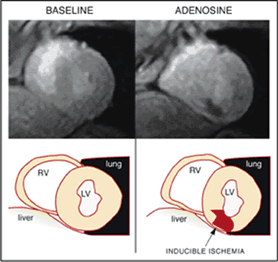
Myocardial perfusion can be detected either by visually analysing the differences in regional myocardial signal intensity of the stress as well as the other images or by performing semiquantitative analysis of the signal intensity curves—while quantitative analysis still remains a difficult goal to achieve. The procedural issues, such as the method for acquiring the images or the semiquantitative approach for analysis, are defined (2) and several studies have demonstrated the value of MR perfusion measurements in assessing the severity and extent of perfusion defects in coronary artery disease.
In patients with suspected coronary artery disease, MRI has shown 91% sensitivity and 94% specificity in the detection of inducible myocardial ischemia, as defined with the use of nitrogen 13 ammonia PET and a sensitivity and specificity of 87% and 85% respectively, when compared to quantitative coronary angiography (3).
Great advantages of MRI are the spatial resolution—which is sufficient to differentiate between subendocardial and subepicardial perfusion, the simplicity of test (takes less than half an hour), the use of non ionising energies, and the reduced operator dependency if a semiquantitative computerised post-processing of images is adopted.
The technique appears to be sensitive enough to evaluate benefits after revascularisation where the improvement of perfusion reserve has been shown to be higher after coronary stenting then after balloon angioplasty alone (4).
Furthermore this new technique will likely open the possibility for assessing myocardial perfusion in a number of challenging pathophisiological situations such as in transplanted hearts or to verify the improvement of regional perfusion after therapeutical procedures such as after intramyocardial implantation of bone marrow cells (5-6).
While multicenter trials are under way to assess the diagnostic accuracy on a large amount of patients, in many Centers, this non invasive approach is already performed routinely as an alternative to the other tests. Furthermore, pilot studies are testing MRI-perfusion as an efficient tool beside the emergency room for the diagnosis of acute coronary syndrome (7).
The promising results obtained up to now, enforce the idea that the evaluation of perfusion by MRI in combination with conventional parameters -especially myocardial function- may further increase diagnostic accuracy in coronary artery disease.
The content of this article reflects the personal opinion of the author/s and is not necessarily the official position of the European Society of Cardiology.


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.