Introduction
Advances in the diagnosis and endovascular treatment of abdominal aortic aneurysm (AAA) patients would not have been possible without the concomitant development in vascular imaging techniques. Duplex ultrasound (DUS), computed tomography angiography (CTA) and magnetic resonance angiography (MRA) can all provide useful information non-invasively.
Depending on what the objective of the exam is, we must define which imaging method will be the most appropriate. The objectives of imaging techniques in AAA are as follows:
- Screening for AAA in patients at high risk
- Surveillance of AAA
- Intervention planning for AAA repair
- Follow-up after AAA repair
Besides the advantages and disadvantages of each imaging modality, the cost of each imaging modality (e.g., purchase, maintenance, use of contrast agents, training of staff, etc.) also plays a role in the use of each technique. Another concern is that, although contrast media provide significant diagnostic benefit, there is some risk of contrast medium-related adverse events in a small percentage of patients, which may include renal failure, contrast-induced nephropathy, and allergic reactions, among others (Table 1) [1,2].
Table 1. Comparison of non-invasive techniques for imaging the aorta.
| DUS | CTA | MRA | |
|---|---|---|---|
| Availability | +++ | +++ | ++ |
| Aortic size | ++ | +++ | +++ |
| Involvement of aortic branches | + | +++ | +++ |
| Cost | + | ++ | +++ |
| Radiation | - | +++ | - |
| Nephrotoxicity | - | +++ | ++ |
| Technical aspects | Operator and patient dependent | Timing of contrast administration important | Unsuitable for ferromagnetic stents and pacemaker bearers. Artefacts |
+ means a positive remark and – means a negative remark.
DUS: duplex ultrasound; CTA: computed tomography angiography; EVAR: endovascular aneurysm repair; MRA: magnetic resonance angiography
The role of imaging for screening and surveillance of AAA
Among the different imaging modalities available for AAA screening and surveillance, ultrasound is the method of choice because it is widely available, time-efficient, inexpensive and accurate [3,4]. Ultrasound can reliably image the infrarenal aorta in 98.5% of subjects; however, visualising the aorta may be difficult in some cases (1-2%) and this should be recognised [5].
The use of a standardised ultrasound protocol reduces variability. Measurements must be performed in a plane perpendicular to the aortic longitudinal axis, which will vary in the presence of aortic tortuosity.
The method by which the aorta is measured is still under considerable debate. The three most widely recognised techniques for measuring the aorta with ultrasound are inner-to inner, outer-to-outer and leading edge-to-leading edge.
The existing literature is unclear as to which method has the best reproducibility, although the inter-observer variability for the antero-posterior measuring plane from outer-to-outer measurement has been reported as lower than the other techniques. AAA definition, based on external ultrasound diameters has been shown to have a sensitivity of 67% and a specificity of 97% in predicting the need for AAA repair within 10 years [1,6].
In addition, diameter measurements vary according to imaging methodology. Therefore, all studies should specify the site and plane of measurement of the aortic diameter.
The main limitations of ultrasound are obesity or excess bowel gas that limits the adequate visualisation of the abdominal aorta, the absence of serial image reconstruction to allow stent graft planning, methodological differences in training and instrumentation, and the fact that there is no visualisation of the thoracic aorta [1,4,5].
Ultrasound should also be used for the surveillance of small AAAs. The optimum frequency for surveillance scans of aneurysms 3.0-5.5 cm in diameter should be stratified according to AAA diameter [1].
For the smallest aneurysms (3-3.9 cm), a three-year surveillance interval is safe. For aneurysms 4.0-4.9 cm in diameter, annual surveillance is safe. Only when the diameter reaches 5.0 cm should the surveillance scans be increased to every 3-6 months (recommendation Class I, Level B) [1].
In case of rapidly growing AAAs (>1 cm/year) or those approaching surgical indications, it is advisable to complete the evaluation by another method - usually tomography, given its major spatial resolution. It is recommended that maximum aneurysm diameter be measured perpendicular to the centreline of the vessel with three-dimensional (3D) reconstructed computed tomography (CT) scan images whenever possible. This approach offers more accurate and reproducible measurements of true aortic dimensions, compared with axial cross-section diameters, particularly in tortuous or kinked vessels where the vessel axis and the patient’s cranio-caudal axis are not parallel [7].
Inter- and intra-observer variability of CT for AAA - defined as Bland-Altman limits of agreement - is approximately 5 mm and 3 mm, respectively. Thus, any change of >5 mm on serial CT can be considered a significant change; however, smaller changes are difficult to interpret. Compared with CT, ultrasound systematically underestimates AAA dimensions by an average of 1-3 mm [7,8].
The drawbacks of CTA consist of administration of iodinated contrast agent, which may cause allergic reactions or renal failure. Also, the cost and the use of ionising radiation limit its use for serial follow-up.
Magnetic resonance imaging (MRI) has the same properties as CTA, but the usefulness of MRI in AAA screening is limited [9], so its indication is triggered by the impossibility of obtaining results by other methods.
Non-invasive techniques for intervention planning
CT and MRI have emerged as the current “gold standards” in the preoperative and postoperative evaluation of AAA. Dedicated aortic imaging is crucial to determine an appropriate repair strategy and for optimal preoperative planning. Operator proficiency and availability of equipment may determine the preferred modality. A prerequisite for a good reconstruction is CTA with 1 mm slice thickness. Multislice computed tomography (MSCT) scanners (16 detectors or higher) are preferred for their higher spatial and temporal resolution compared with lower-end devices [7].
CT plays a central role in the management of AAA when defining the time and type of repair. Its advantages over other imaging modalities include the ability to obtain a complete 3D data set of the entire aorta. This reconstruction allows pre-intervention planning for endovascular aneurysm repair (EVAR) and 3D image fusion of CTA and angiography for real-time operative guidance [9].
As the presence of synchronous aneurysms in other vascular beds may influence surgical decision making, screening of the whole aorta and aorto-iliac segment is advocated. CTA plays a key role in assessing the extent of disease because it provides a complete data set of the entire aorta (including the thoracic aorta) and access vessels, which, with dedicated post-processing software, enables analysis in three perpendicular planes, construction of a centreline, and accurate diameter and length measurement [1,7].
It is important to acknowledge that the measured aortic diameter depends significantly on the method used. Many of the same issues concerning measurement by ultrasound apply to CT measurement, for example, axial versus orthogonal centreline diameters, changes with the cardiac cycle and details of calliper placement.
The feasibility of EVAR and its early and long-term success depend on reliable baseline assessment of aortic morphology, including landing zones for fixation and sealing, and correct measurements for appropriate stent graft selection (Figure 1).
Figure 1. Computed tomography angiography scan multiplanar reformation in a coronal view of the abdominal aorta after endovascular aneurysm repair with the anatomical points to be evaluated prior to the procedure (red arrows).
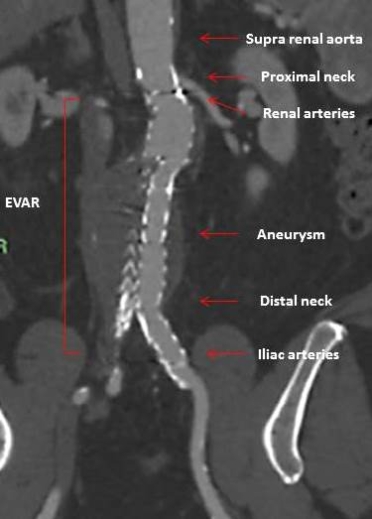
EVAR: endovascular aneurysm repair
The preoperative assessment of AAA includes the measurement of the maximal transverse perpendicular diameter of the aneurysm and its relationship to the renal arteries. Lengths, diameters, angulations, and tortuosity are particularly important for EVAR at the level of the segment of the normal calibre of the aorta, below the renal arteries (proximal neck) and the iliac arteries (distal neck).
CTA will also provide information on venous anomalies, including: the position and patency of the inferior vena cava and left renal vein; organ position, including pelvic or horseshoe kidney; signs of concomitant disease potentially altering prognosis and, thereby, indication for repair (Table 2) [7,8].
Table 2. Main anatomical feasibility information to be evaluated by CTA.
| Anatomical information to be evaluated by CTA | |
|---|---|
| Proximal neck | Diameter, length, shape (cylindrical vs conical), angulation, presence and layout of calcium / thrombus |
| Aneurysm | Diameter, height: renal arteries, distal neck: distance to the renal arteries, iliac bifurcation, presence of developed vessels: inferior mesenteric artery, lumbar arteries and polar arteries |
| Distal neck | Diameter, presence and distribution of calcification and thrombi |
| Primitive iliac arteries | Diameter, calcification, thrombi, angulation, tortuosity, presence of iliac artery aneurysms |
| Other information: Access vessel and lower limb “runoff” vessels/circulation. Concomitant aneurysms in visceral arteries or thoracic aorta. |
|
Although there is no randomised study on the best imaging modality, the consensus is that CTA, including multiplanar and curved 3D vascular reconstructions, is the preferred preoperative imaging modality, if permitted by renal function [10]. Alternatively, MRA may be used for this purpose, even though the assessment of calcification may be more challenging [11], it is more expensive than CT, and there is a less standardised protocol [7,12].
In patients with AAA, the European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines recommended a CTA for therapeutic decision making and treatment planning, and for the diagnosis of rupture (recommendation Class I, Level C) [1].
Intraoperative imaging
Traditionally, digital subtraction angiography (DSA) has been used to ensure correct stent graft deployment and position, and patency of side branches, and to detect the presence of endoleaks. More recently, on-table CT has come to the forefront. The C arm, which includes both the X-ray source and detectors, rotates around the patient creating a 3D set of images similar to CT. Further data are needed before the technique can be recommended in everyday practice [1,13].
Fusion of CTA images with fluoroscopy can be achieved with automatic registration of the preoperative CTA with an intraoperative non-contrast cone beam CT or with a 2D or 3D technique after acquiring fluoroscopic images. “Fusion imaging” has been demonstrated to provide additional real-time 3D guidance with reduced radiation, procedure time, and iodinated contrast doses during complex endovascular repairs. Its value in standard EVAR is, however, limited [1,14].
Post-treatment evaluation of AAA
Imaging after open repair
No randomised studies are available regarding the potential benefit of postoperative imaging surveillance after open surgery repair (OSR) of AAA. Nevertheless, the risk of late para-anastomotic aneurysm and recurrent aortic aneurysm and peripheral aneurysm formation makes it reasonable to consider imaging surveillance of all patients after OSR of an AAA. MRI or CT scanning is the method of choice to detect para-anastomotic aneurysms and new true aortic aneurysms [1].
Imaging after EVAR for AAA
Patients treated by EVAR are more likely to experience aortic complications and reinterventions than those operated on by open surgery. The aim of postoperative imaging is to predict or detect complications. Various imaging modalities can be used during EVAR follow-up. Generally, CTA and/or DUS form the basis for EVAR follow-up imaging.
Early (within 30 days) postoperative follow-up imaging after endovascular aortic repair is required to assess the success of the intervention, and reliable aneurysm exclusion, to detect presence of an endoleak, and component overlaps, and to assess the sealing zone length. An early CTA in addition to clinical examination covers these aspects [15].
Risk factors for post-EVAR instability include graft migration of more than 5 mm, graft kinking or fracture, and persistent endoleaks [16].
An endoleak signifies the presence of flow in the aneurysm sac outside the graft after EVAR. It occurs in up to one third of cases although the prevalence depends on the type of stent graft used as well as the imaging performed during follow-up. The importance of endoleaks is in relation to the risk of AAA rupture related to the pressure to which the aneurysm sac is exposed.
There are five types of endoleak depending on the origin of the leak. Type 1 shows a persistent direct flow in the aneurysm sac due to an inadequate proximal (Type IA) or distal (Type IB) seal of the stent graft. This is dangerous and associated with a high risk of aneurysm rupture. Type II endoleaks originate from collateral vessels (lumbar arteries, inferior mesenteric artery) and are the most common type of endoleak. They can be detected early after EVAR or may occur later during follow-up. Often, these resolve spontaneously and the risk of rupture is low. An endoleak resulting from stent graft component separation or fabric tear is classified as Type III. These endoleaks may occur due to bad deployment of stent grafts with inadequate overlap, proximal or distal stent graft migration, or material fatigue. As with Type I endoleaks, these endoleaks expose the aneurysm to direct aortic pressure with a subsequent risk of rupture. Leakage of blood through the stent graft due to material porosity in the early postoperative period is defined as Type IV endoleak. Endotension (sometimes called Type V endoleak) signifies the presence of sac expansion without any visible endoleak [1,15,16].
CT imaging can be performed either as a single scan (native or arterial phase contrast), two scans (native + arterial phase or arterial + delayed phase contrast), or three scans (native, arterial, and delayed phase contrast imaging). Delayed phase contrast imaging (venous and/or portal sequences) is important to rule out flow in the aneurysm when searching for endoleaks.
MRI can be used in EVAR follow-up in selected patients. Aneurysm diameter measurements can be performed reliably with MR and are comparable to measurements performed with CT. MRI may therefore have a specific role in the imaging of patients with post-EVAR sac growth where CTA is negative or inconclusive. Ferromagnetic stent grafts will result in significant artefacts, which make image analysis difficult [17].
Owing to the risk of graft-related complications and rupture after EVAR, regular imaging follow-up has been regarded as mandatory. Current recommendations regarding regular follow-up suggest up to five CT examinations during the first postoperative year [1]. Despite clear guidelines, follow-up routines vary significantly between centres [18].
Current recommendations regarding screening, planning treatment and follow-up of patients with AAA are summarised in Figure 2.
Figure 2. Non-invasive imaging techniques in different AAA settings.
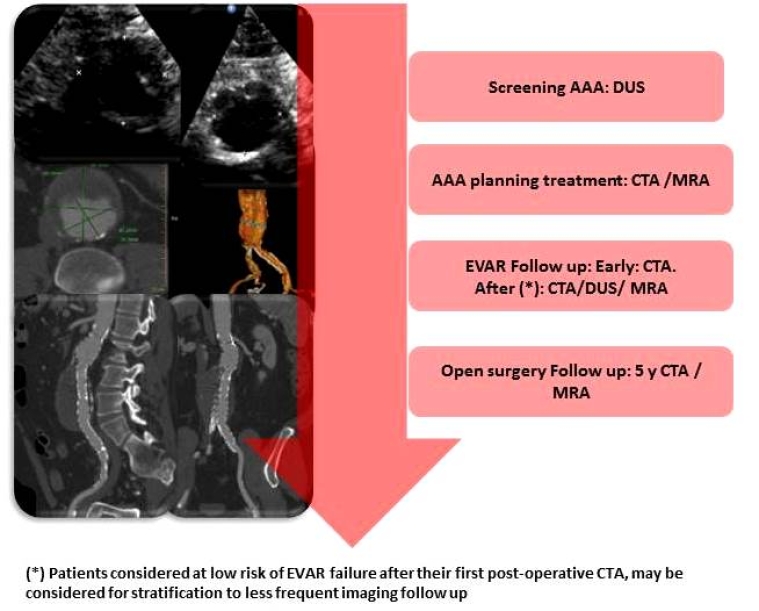
AAA: abdominal aortic aneurysm; DUS: duplex ultrasound; CTA: computed tomography angiography; MRA: magnetic resonance angiography
Utility of positron emission tomography-computed tomography
18F-fluorodeoxyglucose (18F-FDG) positron emission tomography-computed tomography (PET-CT) localises and quantifies metabolic activity of cells, including inflammatory cells, and is a complementary imaging method for the diagnosis and follow-up of aortic pathologies associated with inflammatory aneurysm, aortic infection, infected prostheses and stent grafts [1,19] .
For low-grade graft infection, CT has less accuracy. 18F-FDG PET-CT scanning is a reliable non-invasive imaging modality for the diagnosis and follow-up of prosthetic infection with a sensitivity of 77-93% and a specificity of 70-89% [20].
Imaging of ruptured AAA
Emergency room ultrasound may be useful in identifying the presence of an AAA; however, its sensitivity to detect retroperitoneal haemorrhage is low. As a result, ultrasound cannot be used to identify a leak, but the presence of an AAA in an unstable patient is very suggestive of a ruptured AAA (rAAA). In the endovascular era, another drawback of ultrasound is that it lacks information about anatomical suitability for EVAR. Therefore, an immediate CTA as the key imaging modality advocated for all patients with suspected rAAA [1]. If, however, the patient is not stable enough for a CT scan, he or she should be transported directly to the operating room for emergency surgery.
In haemodynamically stable patients with a suspected ruptured AAA, prompt thoracoabdominal CTA is recommended as the imaging modality of choice (Class I, Level B) [1]
Conclusions
Ultrasonography has rapidly evolved into a cost-effective approach for aneurysm screening while computed tomography is the method of choice when considering a potential therapeutic intervention and for follow-up after AAA repair. The usefulness of magnetic resonance in AAA is limited. Digital angiography has been replaced by CT and its use is reserved for therapeutic purposes. The proper use of each of the imaging techniques allows adequate monitoring and treatment of patients with AAA. However, many challenges still exist in AAA imaging, among which is the inability to accurately identify rupture-prone AAAs.



 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.