Introduction
Patients with aortic stenosis (AS) represent a significant proportion of the current workload of cardiology departments because of the high prevalence of the disease and the development of percutaneous interventional therapeutic options. Decisions that concern therapy in patients with AS are made balancing the operative risk or, recently, the risk of a percutaneous intervention, with the risk of no intervention. Cost is also an issue, but this is beyond the scope of the present article.
While comparing the risks of two therapeutic modes is common practice in cardiology, physicians also need to bear in mind that the prognostic value of symptoms is very important.
What are the Symptoms?
Shortness of breath, angina pectoris and syncope form the triad of symptoms pointing to the need for surgical or percutaneous intervention in severe AS. However, no specific valve area or pressure gradient has been found to predict symptoms [1]. Of this triad of symptoms only syncope can be witnessed or experienced beyond doubt. The subgroup with syncope has the smallest ventricular volumes compared to those with shortness of breath or angina [2]. The threshold of angina or shortness of breath differs significantly among individual patients.
Comorbidities able to produce symptoms similar to those of AS are not uncommon in the age group >70 years who also have a high prevalence of aortic valve calcification and a mild or moderate degree of AS [3]. Conversely, in young adults, symptoms have more definite clinical value and prognostic burden when present in patients with AS.
The distinction between symptomatic patients with severe AS and symptomatic patients with non-severe AS whose symptoms are not related to the valvular lesion is important. The prognosis of the latter group depends on the comorbidities; therefore, the modification of cardiovascular risk factors and management of comorbidities should be monitored together with close follow-up of the deterioration rate of the stenosis [3].
The Measurements
Symptoms are important indicators for intervention but should not influence medical judgement concerning evaluation of the severity of the stenosis.
The meaning of severe AS is not well defined. An aortic valve area (AVA) <1 cm2, a mean gradient >40 mmHg, a peak velocity >4 m/sec, and a velocity ratio <0.25 were considered as the echo criteria defining severe AS. One must keep in mind that the generation of a mean gradient >40 mmHg requires an AVA of 0.8 cm2 not 1 cm2 [4]. Low-flow low-gradient and “paradoxical” low-flow low-gradient severe AS are important clinical entities that can be covered only partially by the previous definition.
A “practical stepwise approach” has been adopted to replace the previous definition. This approach [5] requires flow rate measurement which can be an additional source of error when the left ventricular outflow tract size needs to be measured. Attempts that have been made to simplify the measurements of the severity of AS using a simple measurement such as the velocity ratio seem to create too many grey areas of uncertainty. Atrial fibrillation and suboptimal velocity recordings are also sources of uncertainty. Arterial blood pressure should be measured simultaneously with the velocity measurement [6].
The Role of Exercise Testing
The term “severe” for most clinicians tends to be synonymous with the need for prompt intervention to prevent permanent myocardial damage or sudden cardiac death. However, this concern is not supported by the evidence, because such complications are rare in asymptomatic patients. Symptoms of cardiac origin are valuable pieces of information in the process of decision making. An exercise test is needed first to reveal symptoms of cardiac origin that were not realised and reported previously by the patient, and secondly to depict those patients who, despite their complaints, are able to exercise without symptoms or signs of cardiac origin, such as arrhythmias, blood pressure decline, or electrocardiographic changes. A detailed appraisal of the origin of symptoms also requires an evaluation of functional capacity.
Exercise testing was previously contraindicated in symptomatic patients with AS [4] but was later recommended for “unmasking” symptoms. This was the stimulus for a large-scale study using symptom-limited treadmill exercise (including 683 patients) or bicycle exercises (including 640 patients) [7].
Despite the high percentage of positive exercise tests in these studies, with development of symptoms, arrhythmias and decline of blood pressure (BP) during exercise, severe accidents including death were not reported during the testing itself. Therefore, in the following edition of the European Guidelines the contraindication for exercise testing was no longer emphasised [5].
One could argue that exercise testing in symptomatic patients with AS is not indicated because these patients will in any case be referred to surgery. Still, we have seen that valuable information, such as the presence of coronary artery disease, might well be missing in such patients.
Unfortunately, exercise testing is still underused in AS.
In a more recent report including 316 “apparently asymptomatic” patients who underwent 797 exercise tests, again no deaths were reported during the exercise tests. Symptoms during the test were revealed in 52% of the “apparently asymptomatic” patients and 30 of them died awaiting intervention, but not during the exercise test itself. Authors of this study concluded that, although sudden death may happen in patients with moderate or severe AS, no patient died during exercise testing [8,9]; therefore, it was concluded that the exercise test could be considered safe.
In fact, the prevalence of death during exercise testing cannot be evaluated in AS because very few deaths have been reported. Two asymptomatic patients died during exercise. One of them had left bundle branch block (LBBB) which undermines the diagnostic value of exercise electrocardiography (ECG) [10]. Overall, exercise testing was used in less than 7% of patients with AS, as shown in the Euro Heart Survey [11].
Some patients tend to limit their activities to the point that it keeps them from experiencing symptoms, leading them to be erroneously classified as asymptomatic. Paediatric and young patients do not limit their activities; therefore, symptoms carry a specific burden.
Exercise tests in patients with moderate or severe AS should be closely monitored during the test because, even if they are truly asymptomatic, patients are prone to a vicious pathophysiologic mechanism released because of the fixed obstruction and the increased O2 myocardial demand.
Abnormal stimulation of baroreceptors (the Bezold-Jarisch reflex), hypotension, bradycardia and vasovagal syncope may lead to sudden death. Knowledge of the pathophysiologic mechanism, frequent blood pressure measurement, and close observation of the patient are needed. The appearance of an inappropriate blood pressure increase or decline, bradycardia, arrhythmias, new LBBB or an ST-segment shift should be promptly identified as criteria of the positivity of the test [12].
Symptoms and Exercise Testing in Aortic Stenosis
Exercise testing in AS should be approached as the two faces of Janus because the result of the test could distract us from the correct pathway. On the one hand we see that there are patients who deny that they have any symptoms but who in reality are symptomatic and yet are still sent to the exercise test and, on the other, patients with AS who claim to have symptoms but whose symptoms are not due to the stenotic valve and who would thus not be cured by intervention.
The triad of AS symptoms should be carefully questioned while signs of cardiac decompensation, drop in blood pressure, ECG changes, arrhythmias and exercise intolerance must be inspected. Cardiopulmonary exercise testing enables us to measure O2 consumption during exercise. Despite its limitations, reduced O2 consumption reflects the inability of the heart to deliver O2 to the periphery. This reduction in O2 consumption is related to prognostic parameters and is considered a valuable clinical tool permitting the clarification of the aetiology of shortness of breath (SOB) [13]. However, symptomatic severe AS is considered a contraindication for a cardiopulmonary exercise test [4].
Notwithstanding this, in a study of 101 patients with an AVA <0.6 cm2/m2 and equivocal symptoms in 70% of them, cardiopulmonary exercise had a prognostic value and was seen as safe [14]. Specifically, a cut-off point of 83% of predicted O2 consumption was found to determine prognosis. It was clearly shown that patients who developed symptoms during the test or had decreased O2 consumption reflecting cardiac output and decreased peak O2 pulse which reflects stroke volume may benefit less from intervention, while intervention can be postponed safely in patients with normal O2 consumption [15].
Natriuretic Peptides as a Possible Predictor
There are still a number of unresolved dilemmas in the management of aortic valve stenosis. For instance, how can we detect asymptomatic patients who are at risk of either sudden death or possibly developing “silent” and irreversible ventricular dysfunction?
The inability of patients >70 years of age to undergo exercise testing or cardiopulmonary exercise testing makes the assessment of symptoms controversial and may lead clinicians to suggest intervention at a premature stage without being convinced that this is a timely decision [16]. Interestingly, natriuretic peptides can be used to differentiate heart failure from chronic obstructive pulmonary disease (COPD) in patients presenting with SOB [17]. However, the mechanism of this brain natriuretic peptide (BNP) elevation has not been defined, as plasma levels of natriuretic peptides can increase during exercise in patients with AS. BNP has therefore become an important tool to connect symptomatic status, exercise tolerance, ventricular function and prognosis (Figure 1). In a study of 130 patients with severe AS, it was shown that NtBNP was the only independent predictor of outcome [18].
Figure 1. Doppler tracings and cardiopulmonary exercise test results of a 67-year-old asymptomatic patient who refused intervention. The connection of the severity of the disease with exercise capacity and BNP is shown here during follow-up.
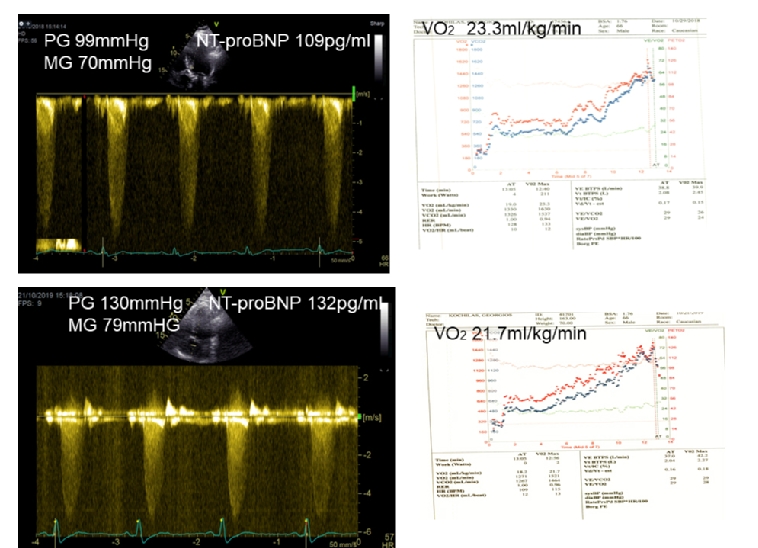
Upper Panel: Baseline
Lower Panel: Changes in 12 months’ time. Peak gradient increased by 31 mmHg, O2 consumption decreased from 23.3 ml/kg/min to 21.7 ml/kg/min and NT-proBNP increased from 109 pg/ml to 132 pg/ml (upper normal 125 pg/ml).
Conclusion
Even though sudden death is much more frequent in symptomatic than in asymptomatic patients [19,20], this does not occur during exercise testing. However, while exercise testing in AS should be monitored carefully it has been seen to be a safe and useful tool for risk stratification and for deciding on the timing of aortic valve replacement (AVR) in patients with severe aortic stenosis.


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.