Introduction
The European Society of Cardiology Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases define peripheral arterial diseases (PADs) as all arterial diseases other than those of the coronary arteries and aorta [1]. This mini-review will focus on atherosclerotic lower extremity artery disease (LEAD), which has been commonly referred to as PAD in the older literature.
LEAD is not an isolated disease of the lower limbs, but rather a manifestation of systemic atherosclerosis that is associated with an increased risk of overall mortality, cardiovascular death and non-fatal ischaemic events [1,2]. As early as 1992, Criqui et al drew the attention of the medical community to the dismal fate of patients with LEAD, noting a threefold increase in overall mortality and an approximately sixfold increase in 10-year cardiovascular mortality [3]. The REACH registry found patients with PAD, i.e., LEAD, to be at higher risk of cardiovascular death, myocardial infarction, stroke or hospitalisation for atherothrombotic events than patients with isolated coronary heart disease or cerebrovascular disease [4,5]. All major guidelines, including the 2016 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice [2], list patients with PAD, i.e., LEAD, in the highest risk category, requiring maximal efforts for cardiovascular disease prevention. In 2001, the PARTNERS programme reported that LEAD was underdiagnosed and undertreated in primary care practices throughout the USA [6], and in 2017 the ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases still emphasise that major efforts are necessary to sensitise healthcare providers, decision makers and the general population about the need for earlier and more efficient prevention and management strategies for the approximately 40 million patients with PADs in Europe [1].
Every practising cardiologist meets patients with LEAD, since many have concomitant cardiac conditions. Recognising LEAD and initiating appropriate management is therefore an essential part of comprehensive cardiological care. In healthcare centres, it is recommended to set up a multidisciplinary vascular team to make decisions on the management of patients with PADs, including LEAD [1].
Prevalence of LEAD
It has been estimated that in 2010 approximately 202 million people were affected with LEAD worldwide, of whom almost 40 million were living in Europe [7]. The number of people with LEAD is increasing, more rapidly in low- and middle-income countries than in high-income countries - with estimated increases between the years 2000 and 2010 of 28.7% and 13.1%, respectively [7].
As with other manifestations of atherosclerosis, LEAD usually presents after the age of 50 years, with an exponential increase after the age of 65 years [1,7]. The prevalence of LEAD reaches approximately 20% by the age of 80 years [1,9]. In high-income countries, symptomatic LEAD is somewhat more frequent in men, although the difference practically disappears in the elderly [1,7,]. In low- and middle-income countries, the prevalence of LEAD is higher in women than in men, especially at younger ages: 6.3% (95% confidence interval [CI]: 4.9-8.1%) vs. 2.9% (CI: 2.0-4.1%) in the 45-49 year category [7].
Risk factors for LEAD
The major risk factors for LEAD are similar to those for coronary and cerebrovascular disease, with some differences in the relative importance of factors [1,8,9]. Smoking is a particularly strong risk factor for LEAD, associated with a 1.9 to 3.4-times higher odds ratio (OR) for developing the disease [8]. Diabetes mellitus is also strongly associated with developing LEAD, with the OR ranging from 1.9 to 4.0, the duration of diabetes playing an important role [8]. Also, LEAD progresses more rapidly to critical limb ischaemia in patients with diabetes mellitus who have a fivefold increased risk for amputation in comparison to non-diabetic patients with LEAD [1,9]. In epidemiological studies, systolic hypertension, elevated total serum cholesterol and decreased HDL-cholesterol were associated with a higher prevalence of LEAD [9]. Obesity, when adjusted for type 2 diabetes, hypertension and hypercholesterolaemia, is not usually associated with LEAD or is even protective [8]. Light to moderate alcohol consumption, in contrast to coronary artery disease, does not seem to be protective against LEAD [8]. Regarding race, African Americans have a 1.5 to 2.0 increased risk of LEAD compared to non-Hispanic whites, even after adjustment for traditional risk factors and inflammatory markers, while Asians seem to have a lower prevalence of LEAD than non-Hispanic whites [8]. The importance of elevated levels of homocysteine for the development of LEAD is controversial, but certainly much less important than previously thought in the 1980s and 1990s [1]. Many studies have reported an association of C-reactive protein and fibrinogen levels with LEAD, but the two are highly correlated [8]. Chronic kidney disease, especially end-stage renal disease requiring dialysis, is associated with a higher incidence of LEAD and poorer outcomes in terms of mortality and limb loss [8]. Data on genetic factors in LEAD are limited and are an active area of investigation [8].
Clinical presentation and diagnosis of LEAD
LEAD has several different presentations, categorised according to the Fontaine or Rutherford classifications into asymptomatic disease, non-disabling (mild or moderate) claudication, disabling (severe) claudication, ischaemic rest pain and ulceration or gangrene (minor or major tissue loss) [1,9]. Symptoms and their intensity may vary from one patient to another even if the morphologic features of arterial obstruction are similar [1]. Many patients who do not have typical claudication symptoms and might be considered asymptomatic already have impaired walking ability [1].
In diagnosing LEAD, clinical history and physical examination that includes systematic palpation of peripheral pulses should be supplemented by determination of the ankle-brachial index (ABI), i.e., the ratio of the ankle systolic pressure and the brachial systolic pressure [1]. An ABI <0.90 has 75% sensitivity and 86% specificity for diagnosing LEAD [10]. Its sensitivity is poorer in patients with diabetes or end-stage chronic kidney disease because of poorly compressible calf arteries due to calcification of the arterial media [1]. The ESC Guidelines on PAD recommend determining the ABI in all patients with a clinical suspicion of LEAD, patients at risk of LEAD because of other atherosclerotic diseases (such as coronary or carotid disease) or abdominal aortic aneurysm, chronic kidney disease or heart failure, as well as in screening of all people over 65 years of age, people under 65 years of age who have high cardiovascular risk, and people above 50 years of age who have a family history of LEAD [1].
When revascularisation is considered, arterial anatomy and haemodynamics may be assessed by duplex ultrasound, and morphologic information can be supplemented by computed tomography angiography or magnetic resonance angiography, while digital subtraction angiography is primarily intended for guiding percutaneous revascularisation procedures [1].
Fate of the affected limb and fate of the patient with LEAD
Regarding the fate of the affected limb, the TASC II guidelines from 2007 state that over a five-year period 70-80% of symptomatic LEAD patients remain stable, claudication worsens in 10-20%, and 5-10% (i.e., 1-2% per year) progress to critical limb ischaemia [9]. A recent meta-analysis reported a higher, approximately 21% five-year rate of progression of intermittent claudication to critical limb ischaemia (CLI) with 4-27% of CLI patients undergoing amputations [11].
Regarding the overall prognosis, both the TASC II guidelines and the recent meta-analysis agree that the cardiovascular mortality of patients with symptomatic LEAD over five years is more than double compared to the reference population, although the absolute numbers tend to be decreasing [1,9,11].
Treatment of LEAD is “local” and “systemic”
Treatment of patients with LEAD always includes two aspects, the local one and the systemic one: 1) addressing limb-related symptoms, which are primarily managed by exercise training in non-disabling claudication and by revascularisation in disabling claudication and critical limb ischaemia [1,9], and 2) general cardiovascular prevention measures, that are primarily intended to reduce the incidence of myocardial infarction and ischaemic stroke, but are also beneficial for the fate of the affected limb [1,2].
The guideline recommendations for cardiovascular disease prevention in patients with LEAD are essentially the same as for all other forms of atherosclerotic vascular disease [1,2]:
- Smoking cessation is recommended (Class I, level B).
- Healthy diet and physical activity are recommended (Class I, level C).
- Statins are recommended in all patients (Class I, level A) with the goal of reducing LDL-cholesterol to <1.8 mmol/l (70 mg/dl) or decreasing it by >50% if baseline values are 1.8-3.5 mmol/l (70-135 mg/dl) (Class I, level C).
- In diabetic patients, strict glycaemic control is recommended (Class I, level C).
- Antiplatelet therapy is recommended in all patients with symptomatic PADs (Class I, level C).
- In patients with PADs and hypertension, it is recommended to control blood pressure at levels <140/90 mmHg (Class I, level A). Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers should be considered as first-line therapy in patients with PADs and hypertension (Class IIa, level B).
What is achieved by following the guidelines – implications for prevention
In primary prevention of LEAD, the most important strategy is to avoid all known modifiable risk factors. Among the original cohort of the Framingham Heart Study, claudication developed in 381 men and women during 38 years of follow-up [12]. The risk for intermittent claudication increased with elevated serum cholesterol (OR increase of 1.2 for each 1 mmol/l, i.e., 40 mg/dl), cigarette smoking (OR increase of 1.4 for every 10 cigarettes smoked per day), hypertension (OR 1.5 for mild and 2.2 for moderate hypertension), and diabetes mellitus (OR 2.6) [12].
Concerning secondary cardiovascular prevention, most of the guideline-recommended interventions are based on high-quality clinical studies evaluating one particular intervention. Only recently, studies in patients with LEAD have addressed the issue of following guideline-recommended cardiovascular prevention as a whole [13-15].
Hussain et al have compared a cohort of 290 patients with symptomatic LEAD who were enrolled in systematic assessment of vascular risk (SAVR) at a single tertiary vascular centre in Ontario, Canada, with 501 propensity-matched patients undergoing the usual care at other tertiary centres. Systematic attention to antiplatelet agents, statins, angiotensin-converting enzyme inhibitors, blood pressure control, lipid control, diabetic glycaemic control, smoking cessation and target body mass index resulted after seven-year follow-up in a significantly lower hazard ratio (HR) of 0.63 (CI: 0.52-0.77) for the composite primary outcome of death, myocardial infarction or ischaemic stroke [13]. Also, the SAVR patients were less likely to undergo major amputation of the lower limb (HR 0.47, CI: 0.29-0.77), minor amputation (HR 0.26, CI: 0.13-0.54), peripheral bypass surgery (HR 0.47, CI: 0.30-0.73), or hospitalisation due to heart failure (HR 0.73, CI: 0.53-1.00). The rate of peripheral angioplasty was higher among the SAVR group (HR 2.97, CI: 2.15-4.10) [13].
Similarly good results were reported by Höbaus et al from Vienna, Austria, who for five years followed 370 patients with LEAD treated intensively by a vascular medicine group in a tertiary centre compared to 332 patients with LEAD, comparable in terms of baseline characteristics, who were treated by their primary care physicians [14]. Meticulous attention to smoking status, protective medication, blood pressure control, lipid control and glycaemic control, as well as timely peripheral revascularisation in case of worsening LEAD symptoms, resulted in a 90.8% five-year survival of intensively treated patients in comparison to 66% five-year survival of the usual-care group [14].
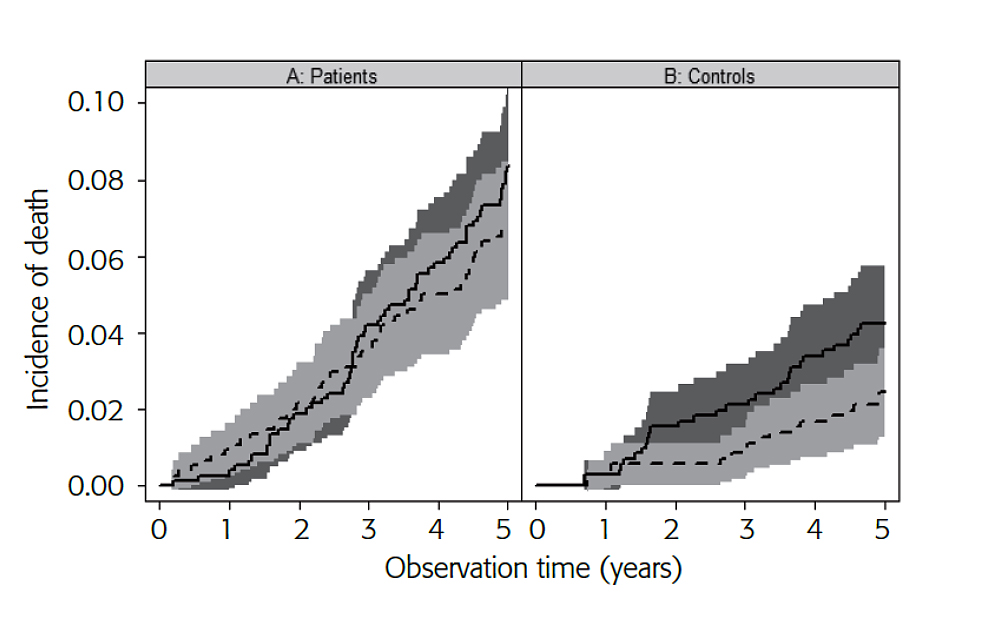
Slovenian researchers have shown that very good outcomes of LEAD patients are possible also in the primary care setting after special training of the participating physicians [15]. A cohort of 742 patients with LEAD and 713 age- and sex-matched control subjects without LEAD were managed for five years according to the European guidelines on cardiovascular disease prevention and evaluated yearly for occurrence of death (stratified into cardiovascular and non-cardiovascular causes), non-fatal acute coronary syndrome, ischaemic stroke and critical limb ischaemia (major ischaemic events) and revascularisation procedures (minor events). In the LEAD group, the five-year survival was 84.7% (CI: 82.1-87.3) and the proportion of cardiovascular deaths did not differ significantly from non-cardiovascular deaths (6.9 vs. 8.4%, p=0.14) (Figure 1A). In the control group, the five-year survival was 93.3% (CI: 91.5-95.2%) and cardiovascular deaths were less frequent than non-cardiovascular deaths (2.4 vs. 4.3%, p=0.05) (Figure 1B). The groups differed in five-year major event-free survival, i.e., 76.7% (CI: 73.7-79.8%) in LEAD vs. 89.9% (CI: 87.7-92.2%) in controls, p<0.001, and in event-free survival, i.e., 56.2% (CI: 52.7-59.9%) in LEAD vs. 82.4% (CI: 79.9-85.3%) in controls, p<0.001, with the major difference in minor events resulting from peripheral angioplasties in LEAD patients [15]. It is noteworthy that, with rather similar baseline characteristics of the participating subjects, the major event-free survival of patients in the Slovenian primary care settings was very similar to the numbers reported by Hussain et al after five years for intensively treated LEAD patients in the tertiary vascular centre in Ontario, Canada, and the overall survival was closer to the patients treated by the Vienna vascular medicine group than by the Austrian general practitioners [13-15].
Figure 1. Competing risk analysis for patients with LEAD undergoing guideline-directed cardiovascular prevention (A) and control subjects (B) for risk of death from cardiovascular causes (dashed line) and from non-cardiovascular causes (solid line), expressed as proportion of the observed cohort. 95% confidence intervals are given as shaded areas. In patients with LEAD undergoing cardiovascular prevention, the incidence of cardiovascular death and non-cardiovascular death did not differ significantly (p=0.14), while in control subjects the incidence of cardiovascular death was marginally significantly lower than that of non-cardiovascular death (p=0.05). (Reproduced with the permission of Edizioni Minerva Medica [15])
Directions for further improvement
Although guideline-directed care offers the best chance for patients with LEAD, the high atherosclerotic burden in several vascular territories inevitably results in “residual” ischaemic events. Apart from rigorous adherence to guideline recommendations, further improvements in treatment are needed. Recently, additional benefits for patients with symptomatic LEAD were reported with rigorous LDL-cholesterol lowering [16] and by supplementing antithrombotic treatment with low-dose aspirin with low-dose rivaroxaban [17,18].
Among 27,564 patients with atherosclerotic vascular disease in the FOURIER trial, there were 3,642 patients with LEAD, defined as having intermittent claudication and an ankle-brachial index of <0.85 or having had a prior peripheral vascular procedure [16]. Patients who were already on statins were randomised into treatment with the PCSK9 inhibitor evolocumab or placebo. Lowering LDL-cholesterol to very low levels (median 0.8 mmol/l, i.e., 31 mg/dl) with evolocumab significantly reduced major adverse cardiovascular events in patients with symptomatic LEAD: the HR for a composite of cardiovascular death, myocardial infarction, stroke, hospital admission for unstable angina, or coronary revascularisation was 0.79 (95% CI: 0.66-0.94), p=0.0098, compared to placebo. Evolocumab also reduced the risk of major adverse limb events in all patients (HR 0.58, 95% CI: 0.38-0.88; p=0.0093) with consistent effects in those with and without known LEAD [16]. These data support the concept of reducing LDL-cholesterol levels to very low levels in all patients with symptomatic LEAD [16].
In the COMPASS trial, 27,395 patients with stable atherosclerotic vascular disease were randomised regarding antithrombotic treatment to receive rivaroxaban 2.5 mg twice daily plus aspirin 100 mg once daily, rivaroxaban alone 5 mg twice daily, or aspirin alone 100 mg once daily [17]. The superiority of rivaroxaban plus aspirin was demonstrated in comparison to aspirin after a median follow-up of only 23 months with an HR of 0.76 (CI: 0.66-8.86), p<0.001, for the primary outcome - a composite of cardiovascular death, stroke, or myocardial infarction [17]. More major bleeding events occurred in the rivaroxaban-plus-aspirin group, but there was no significant difference in intracranial or fatal bleeding between these two groups. Rivaroxaban alone did not result in better cardiovascular outcomes than aspirin alone, but resulted in more major bleeding events [17]. Among the enrolled patients in the COMPASS study, there were 7,470 patients with PADs, i.e., 4,129 patients with symptomatic LEAD, 1,422 patients with coronary disease and ankle-brachial index <0.90, and 1,919 patients with >50% carotid artery stenosis or prior carotid revascularisation [18]. In the PADs subgroup, rivaroxaban-plus-aspirin in comparison to aspirin alone reduced the HR for the primary outcome to 0.72 (CI: 0.57-0.90), p=0.005, reduced the HR for major adverse limb events to 0.54 (CI: 0.35-0.84), p=0.005, and reduced the HR for major amputation to 0.30 (CI: 0.11-0.80), p=0.01. Major bleeding was increased, but not fatal or intracranial bleeding, so there was a net clinical benefit with a combined HR of an adverse event in comparison to aspirin alone of 0.72 (CI: 0.59-0.87), p=0.0008 [18]. These results support the supplementation of low-dose aspirin by low-dose rivaroxaban in patients with LEAD or carotid artery disease.
Conclusion
LEAD is a prevalent, often neglected manifestation of atherosclerosis that should be recognised by all practising cardiologists. Strict cardiovascular prevention according to guidelines saves lives, reduces non-fatal atherothrombotic events and improves the fate of the affected limb in patients with LEAD.


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.