Introduction
Transcutaneous techniques have revolutionised the management of aortic stenosis (AS) [1]. The original PARTNER trial data demonstrated the effectiveness of transcatheter aortic valve implantation (TAVI) in patients without surgical options, and the equivalence of TAVI in high-risk scenarios [2,3]. More recently, the effectiveness of TAVI (particularly delivered by the femoral route) in intermediate-risk patients has been demonstrated [4,5]. This is changing the paradigm of care from “surgery first until the risks become too high” to “optimum strategy to give the best result in the individual patient". This is reflected in the most recent ESC/EACTS guidelines (2017). Both surgery and TAVI have their merits. A better understanding of these allows an individualised decision-making process to get the best long-term outcomes. Amongst patients at high and low risk, the treatment algorithms are clear. This article addresses patients who have AS which is not degenerative or has other associated valvular lesions and who require a more individualised approach.
Bicuspid disease
Contemporary estimates suggest that 0.5% to 2% of the population have a bicuspid aortic valve with a strong male preponderance [6]. It is associated with more rapid development of degenerative lesions (stenosis or regurgitation). Consequently, younger cohorts have a high proportion of bicuspid cases, typically between 40 and 50%. However, bicuspid disease is present in up to 20% of patients over the age of 80 [7]. It can be subtle to diagnose, particularly when the fusion of the two cusps forms a fibrous, or even calcific, raphe (Figure 1). Both echocardiography and computed tomography (CT) can be misleading [8], particularly when the valve is examined in diastole (closed), where it can be difficult to distinguish a raphe from a thickened cusp margin. Examining systolic frames and the orifice shape is strongly recommended [9].
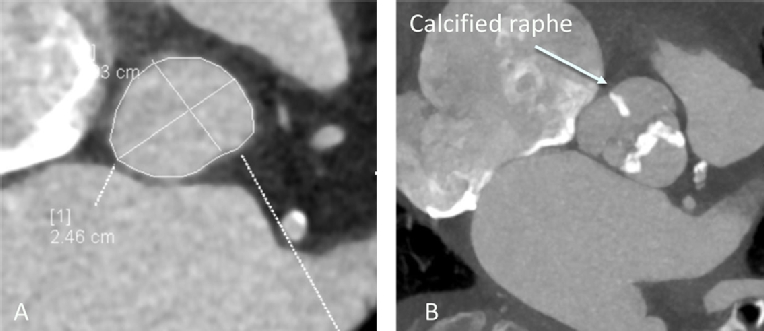
Figure 1. A) Gated CT demonstrating accurate assessment of annular asymmetry. B) Systolic gated CT frame at the level of the aortic valve demonstrating calcification of the raphe dividing the non-coronary and right coronary cusps. This can easily be misdiagnosed as a tricuspid valve.
The anatomy poses significant potential problems for TAVI. The virtual aortic valve annulus is both large and asymmetric (Figure 1), leading to more complicated valve sizing and the potential risk of regurgitation, which is poorly tolerated in AS [10]. The valve may be less calcified, giving a higher risk of valve displacement. The presence of aortopathy in up to 70% of cases [11,12] is an additional consideration, especially where the aortic dimensions exceed 4.5 cm [13]. Emerging valve design and pre-procedural planning should address these limitations.
Valve sizing
Three-dimensional (3D) techniques are essential for valve sizing. CT is the preferred imaging modality because of its isotropic voxel size and clear tissue interfaces. This allows a simultaneous assessment of valve annulus dimension and eccentricity, leaflet and subvalvular calcification, coronary height, and the size of the ascending aorta. Where CT is either unavailable or contraindicated, 3D transoesophageal echocardiography (TOE) provides a well-validated alternative [14].
Outcome of interventions
Bicuspid valves have been excluded from the randomised clinical trials of TAVI. Outcome and effectiveness data therefore come either from single site experience or registries, most of which have relatively small numbers. Mortality after TAVI does not seem to be affected by the presence of a bicuspid valve. Only in the Milan experience was late mortality higher, but this was explained by features not related to the valve implant [15]. The risk of aortic regurgitation using first- and second-generation TAVI valves is higher. The exact risk is difficult to define because of a lack of unified definitions. In the TAV-in-BAV registry, the risk of post-procedural significant AR was 28%, although this dropped with CT-based annular sizing [16]. Yoon and colleagues report a much lower risk of significant aortic regurgitation, although this was still higher in the bicuspid patients (10.4% vs. 6.8%). This suggests a difference in definition. This registry confirms a small but significant risk of complications including conversion to surgery and second valve implants. It also confirms that third-generation percutaneous devices have a lower risk of paraprosthetic regurgitation [17]. While the effectiveness of surgical valve replacement is not in doubt, surgical advances such as the sutureless valve appear to be equally effective in bicuspid disease [18].
Take home messages
- In the absence of randomised evidence, surgical replacement should be the standard of care for patients with bicuspid disease, in particular if the ascending aortic dimensions exceed 4.5 cm.
- Where patient factors predict a very high surgical complication risk, TAVI with current techniques is an acceptable alternative.
- TAVI requires careful attention to valve sizing using 3D techniques and the availability of a range of devices to minimise the risk of post-procedural aortic regurgitation and other complications.
Mitral regurgitation
Patients with AS frequently have coexistent mitral regurgitation (MR). In the PARTNER series of studies, this was observed in more than 20% of patients [19]. This can be due to primary disease of the mitral valve (PMR) or secondary restriction due to changes in annular shape or ventricular geometry (functional mitral regurgitation [FMR]). Increasingly, both primary and secondary elements are present, which makes true dichotomisation challenging.
Pathophysiology
Changes in ventricular geometry, systolic atrioventricular pressure gradients, and the mitral annular relations mean that the effect of the relief of AS is unpredictable. While initial reports from the PARTNER study suggested that patients undergoing TAVI did better than those undergoing surgical valve replacement [19], most studies have demonstrated that the presence of MR predicts an adverse outcome compared with those who have only minor or no regurgitation. In a meta-analysis of 13 studies, Sanino et al [20] demonstrated a mortality deficit at 30 days and at both one and two years, although the study did demonstrate an improvement in MR. Similar findings were observed in the meta-analysis of Chakravarty et al [21], where the overall excess risk attributable to MR at 30 days was 35%. Interestingly, there was no difference between secondary and primary MR in this study. In a single study which opposes these findings, Vollenbroich et al demonstrated a significantly higher mortality amongst those with FMR [22] as well as other markers suggesting adverse remodelling and function such as lower mean transvalvular gradients. Nombela-Franco et al [23] demonstrated that a large proportion of patients with FMR undergoing surgical aortic valve replacement demonstrate an improvement when MR was at least moderate. Similarly, patients with moderate or severe MR undergoing TAVI showed improvement in up to 50% (in those with moderate or more MR), although the results for all grades of MR were more ambiguous.
Assessment of mitral regurgitation
Given the complex nature of both primary and secondary MR in this context, adequate assessment before a proposed procedure is essential. This should be carried out using TOE with the availability of 3D imaging. TOE allows a detailed assessment of valve morphology, in particular subtle degenerative changes that may not be observed during TTE. In FMR, an assessment of valve tenting, leaflet restriction and annular dimensions may predict an adverse response to surgical annuloplasty. The severity of MR during TOE is only useful if severe, especially in FMR, as changes in loading conditions may lead to erroneous underestimates of MR severity. TOE also allows an assessment of the suitability for percutaneous mitral valve edge-to-edge repair in the event of ongoing clinically relevant regurgitation for those in whom further surgery is not feasible [24].
Take home messages
- In patients with PMR which is significant, and in whom surgical risks are not preclusive, surgical aortic valve replacement with either repair or replacement of the mitral valve is the optimum strategy.
- For secondary mitral regurgitation, the relative contribution of anatomical vs. physiological drivers (such as marked ventricular remodelling or severe valve tenting) should be taken into consideration and quantitative assessment of mitral regurgitation severity should be undertaken.
- In severe secondary MR, improvement is seen in 50% of cases and no change in the remainder.
- TAVI or SAVR in isolation remains a reasonable option in FMR as there is no randomised evidence of benefit from the surgical repair/replacement of bystander MR.
- In inoperable patients, an assessment for the suitability of percutaneous edge-to-edge valve repair should be undertaken if TAVI fails to bring about a successful clinical result.
Radiation-induced aortic valve disease
The widespread use of radiation to treat both solid tumours and haematological malignancy in the thorax results in significant toxicity. While radiation sparing techniques are now widespread, the results of radiation exposure are delayed, by up to twenty years [25]. Those presenting today may have had highly significant exposure in the late 20th century. Fibrotic toxicity affects all structures within the beam including not only the aortic and the mitral valves, but also the myocardium, pericardium, and aorta, as well as causing severe aortic calcification and pulmonary fibrosis. This means that the prognosis and risk associated with patients having cardiac surgery is significantly higher than in equivalent patients without radiation exposure [26].
Patient evaluation can be advanced before the role of radiation is recognised. Echocardiographic features are very typical: in particular, heavy thickening of the aortic/mitral continuity and anterior mitral leaflet should alert the assessor to the possibility of radiotherapy (Figure 2).
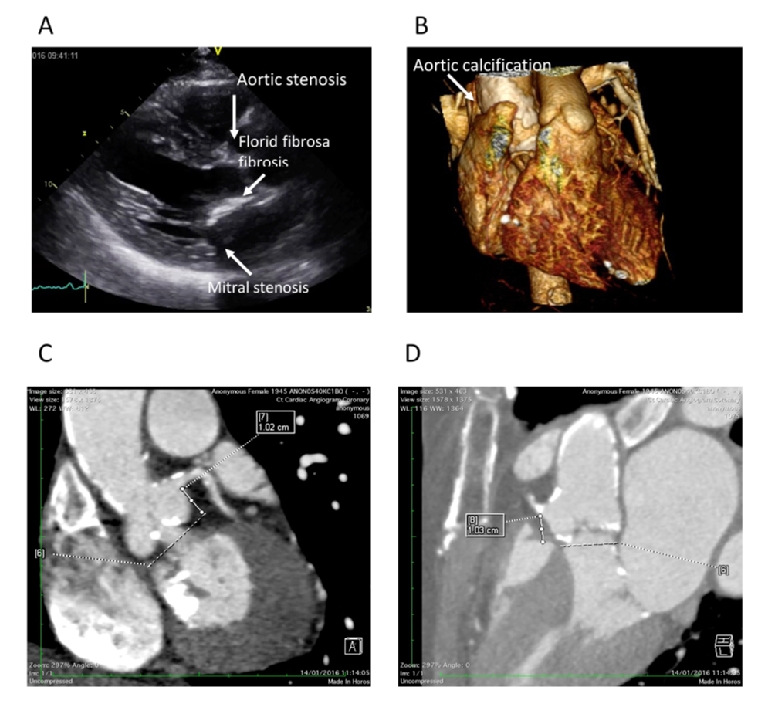
Figure 2. A) Parasternal long-axis view demonstrating the typical features of radiation-induced heart valve disease. B) 3D volume rendering demonstrating severe circumferential aortic calcification. C) & D) Extensive valve and proximal coronary artery calcification and valvular calcification.
Assessment of radiation-induced heart disease
Evaluation of patients is more detailed than in those with conventional aortic valve disease. A multimodality approach is always required. Assessment of the severity of the aortic valve stenosis is best undertaken using echocardiography. The standard measures of severity apply. However, it is common for patients to express either low flow, or paradoxical low flow pattern (with a transaortic flow rate of less than 35 mls/m2) because of radiotherapy-induced myocardial dysfunction. When the ejection fraction is preserved, significant LV dysfunction can still be demonstrated by careful attention to longitudinal function, in particular global longitudinal strain (27).
Gated CT evaluation of the chest is mandatory. This offers an opportunity to confirm the severity of the aortic valve stenosis, although using the aortic valve Agatston score is not validated in this context. The proximal coronary arteries can be examined, although the high level of calcification at and around the aortic root may make accurate assessment of the coronary difficult, signalling the need for invasive angiography. The CT assessment should include the thoracic aorta to describe the extent and location of aortic calcification and how this may affect surgical access. Finally, using the appropriate lung windows an assessment of the location and extent of pulmonary fibrosis should be undertaken.
In contradistinction to their high surgical mortality, patients with radiation-induced aortic valve disease appear to do as well as those with degenerative aortic stenosis after TAVI [28]. Mitral stenosis is also more common and observed in over 50% of cases but, despite this, the functional improvement is equivalent [29].
Take home messages
- Patients with chest radiation exposure have a significant delayed risk of AS.
- Pathology is rarely confined to the aortic valve; the mitral valve is frequently involved, as are the proximal coronary arteries and the ascending aorta.
- Surgery is at considerably higher risk.
- Assessment should be made in a centre with considerable expertise. The availability of gated CT and both 2D and 3D echocardiography is essential.
- TAVI is associated with a high level of technical and clinical success and is the preferred treatment strategy, assuming mitral valve disease does not dominate the clinical presentation; the risk of post-procedural mitral stenosis remains higher.
Conclusions
AS remains a common disease which requires timely and appropriate therapy. The emerging evidence base supporting the long-term clinical efficacy and durability of TAVI has resulted in a philosophical change, highlighted in the 2017 ESC/EACTS guidelines. These shift the emphasis from age alone in favour of selecting the most appropriate technique for the best long-term results mediated by the multidisciplinary Heart Team. The guidance points towards certain factors that should sway clinical decision making. The circumstances outlined in this article are cases in point and all highlight the need for meticulous diagnostic work-up. Integrating detailed echocardiography and the cross-sectional imaging from CT will assist the Heart Team to make the most appropriate management recommendations.
Sponsored by:




 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.