Introduction
Pericardiocentesis is the most useful therapeutic procedure for the early management or diagnosis of large, symptomatic pericardial effusion and cardiac tamponade. [1] The first description of cardiac decompression was in 1653, when Riolanus suggested sternal trephination to relieve pericardial pressure. In 1911, Marfan first described the subxiphoid approach, which had been used for the blind pericardiocentesis procedure for decades, despite the significant morbidity and mortality rates (50% and 6%, respectively). [2] In subsequent years, the techniques recommended for safe and successful pericardiocentesis have changed considerably, particularly with the introduction of fluoroscopic, electrocardiographic and, finally, echocardiographic guidance, [3] and with the description of approaches other than the substernal one (apical and parasternal).
Cardiac tamponade
Cardiac tamponade is a life-threatening slow or rapid compression of the heart due to increasing pericardial fluid. The pericardium can stretch to accommodate fluid accumulation, but when the pericardial compliance cannot increase any further, an equalisation of intrapericardial pressures (central venous, right and left cardiac chambers) occurs, typically around 15-20 mmHg. At this point, the right ventricle collapses and hypotension becomes severe. The haemodynamic effect of significant pericardial effusion is a continuum because a small increase in pericardial content couples the pericardium to the heart, significantly increasing atrial and especially ventricular interaction. Such phenomena exaggerate the normal reciprocal respiratory effects on the right and left sides of the heart. In the first steps of the haemodynamic impairment of pericardial effusion, patients may show only echocardiographic findings of heart chamber compression (right and left atrial collapse, right ventricle collapse, swinging heart, leftward of interventricular septum motion, inferior vena cava congestion) without clinical signs and symptoms (subclinical tamponade). In these cases, echocardiographic findings may be too sensitive and overdiagnose cardiac tamponade in patients with only subtle evidence of haemodynamic compromise. Therefore, echocardiographic tamponade is not a clear indication of pericardiocentesis. [4]
Clinically, cardiac tamponade is defined as the decompensated phase of cardiac compression, resulting from increased intrapericardial pressure. Clinical symptoms and signs include dyspnoea, elevated jugular venous pressure, hypotension, tachycardia, and pulsus paradoxus. At least one of these is present in over 75% of cases. [4]
Effect of pericardiocentesis
The effect of pericardiocentesis is often immediate: the drainage of a few millilitres of the effusion significantly increases stroke volume, reduces intrapericardial and atrial pressures, and permits separation between right and left filling pressures. Tachycardia and dyspnoea decrease, whereas arterial pressure increases and pulsus paradoxus disappears.
Indications of pericardiocentesis
In haemodynamically unstable patients, an emergent procedure is mandatory because only the removal of fluid allows a normal ventricular filling and restores an adequate cardiac output. [1,3,5] Otherwise, the procedure could be performed within hours following presentation and the most appropriate visual guidance and approach could be planned. A scoring index has recently been proposed in patients with a suspicion of tamponade for deciding whether to perform urgent pericardiocentesis or drainage later in subsequent hours. It consists of three components obtained at initial presentation: aetiology, clinical presentation, and echocardiographic findings. [6]
In case of pericardial effusion without haemodynamic compromise, pericardiocentesis is indicated for symptomatic moderate to large effusion non-responsive to medical therapy, or in case of a smaller effusion, when tuberculous, bacterial or neoplastic pericarditis is suspected, or in case of chronic (lasting more than three months), large pericardial effusion (>20 mm on echocardiography in diastole). [1]
Pericardiocentesis for diagnostic purposes is not justified in cases of mild or moderate effusions (<20 mm) for the following reasons: 1) low diagnostic power (the underlying pathology is often already known or identifiable through different non-invasive tests); 2) viral (idiopathic) pericarditis is usually self-limiting and only requires an anti-inflammatory treatment; and 3) high procedural risk compared with low diagnostic yield. [7]
Contraindications
There are no absolute contraindications to pericardiocentesis when cardiac tamponade and shock occur. Aortic dissection and post-infarction rupture of the free wall are contraindications to needle pericardiocentesis (surgical tamponade) due to the potential risk of aggravating the dissection or myocardial rupture via rapid pericardial decompression and restoration of systemic arterial pressure. However, if surgical management is not immediately available, or if the patient is too unstable, pericardiocentesis and drainage of very small amounts of the haemopericardium can be attempted in order to maintain blood pressure at around 90 mmHg as a bridge to emergency surgery. [8] Relative contraindications include uncorrected coagulopathy, anticoagulant therapy, thrombocytopaenia (PLTc <50,000/mm3).
Approaches
Fluoroscopy-guided technique
The fluoroscopic approach was the first imaging system used for percutaneous pericardiocentesis. It is performed through the subxiphoid approach with a needle containing a contrast medium, directed towards the left shoulder at an angle of 30° to the skin. Needle position in the pericardial space is confirmed by the contrast agent medium injection: the inferior position appearance of a sluggish layering of the contrast medium indicates the correct position and that a soft J-tip guidewire can be introduced. It is essential to check the guidewire position in at least two angiographic projections (lateral view and anterior-posterior view). [9]
This procedure is standardised and effective, but it can only be performed in heart catheterisation laboratories and it implies an exposure to radiation for both the patient and the physician. However, this procedure could be very useful to treat iatrogenic tamponade during percutaneous procedures. An echocardiographic examination to assess the distribution and amount of pericardial effusion should always precede the fluoroscopy-guided procedure, whenever possible.
Computed tomography-guided technique
In recent years, evidence of the feasibility of pericardiocentesis under computed tomography (CT) guidance has been reported. Through the planning CT scan, the full extension of the pericardial effusion is evaluated and the optimal entrance point is defined and marked on the skin. Next, the needle is advanced into the pericardial effusion and a single CT scan is used for verification of the needle position. This technique does not allow a continuous visualisation of the needle and it implies a significant exposure to radiation for the patient. In addition, it is not widely available and logistically feasible, and is time-consuming (median duration of 65 minutes). [10] However, it can be successfully used in patients with a poor ultrasound window and can show loculated effusion very effectively, allowing identification of the best entry site. Moreover, a CT scan can measure the density of the pericardial effusion, thus avoiding a procedure failing in case of highly viscous effusions, such as purulent ones and intrapericardial haematoma. [9] Finally, a CT scan allows assessment of the entire chest and detection of associated abnormalities, which is useful in identifying the underlying disease. [11]
Echo-guided technique
Echocardiography-guided pericardiocentesis is a safe and simple technique, introduced at the Mayo Clinic in 1979 and widely used nowadays. [3] The echocardiography-guided approach allows defining the position of the effusion, the ideal entry site and needle trajectory for pericardiocentesis. There are two different approaches to echo guidance: the first (described by the Mayo Clinic) is the echo-assisted method, in which the operator memorises the optimal needle trajectory and advances the needle towards the pericardial space without a continuous ultrasound visualisation. [3] The second approach is the echo-guided method with a continuous echocardiographic monitoring. It has also been proposed to use a needle carrier mounted on the ultrasound transducer to advance the needle to the pericardial space. [5]
Puncture site
Three main approaches can be used for pericardiocentesis: the apical, the subcostal or the parasternal approach.
Traditionally, a subcostal approach has been preferred, largely because it was considered the safest route without image guidance. However, pericardial effusion is not always circumferential and equally distributed; consequently, an ultrasound evaluation of the ideal entry site for drainage is fundamental for procedural success. The Mayo Clinic advocates selecting the approach based purely on echocardiographic findings and defines the optimal entry site as the point where the pericardial space is closest to the probe and the fluid accumulation is maximal, with no intervening vital organs. This site is more often para-apical than subcostal. [3] Furthermore, an observational series on echo-guided pericardiocentesis demonstrated a greater success rate and a minor complication rate when the entry site was echocardiographically selected rather than when the subxiphoid approach was routinely used (Table 1, Figure 1). [3,5,12].
Table 1. Characteristics of the Different Pericardiocentesis Approaches
|
Place of Puncture |
Description |
Disadvantages |
Advantages |
|---|---|---|---|
|
Apical |
The needle insertion site is 1-2 cm lateral to the apex beat within the fifth, sixth or seventh intercostal space. Advance the needle over the superior border of the rib to avoid intercostal nerves and vessels. |
Risk of ventricular puncture due to the proximity to the left ventricle. Increased risk for pneumothorax for the proximity to the left pleural space. |
The thicker left ventricle wall is more likely to self-seal after puncture. Due to ultrasound not penetrating air, using echocardiographic guidance ensures avoidance of the lung. The path to reach the pericardium is shorter. |
|
Parasternal |
The needle insertion site is in the fifth left intercostal space close to the sternal margin. Advance the needle perpendicular to the skin (at the level of the cardiac notch of the left lung). |
Risk of pneumothorax and puncture of the internal thoracic vessels (if the needle is inserted more than 1 cm laterally). |
Echocardiographic guidance, also with phase array probe, provides a good visualisation of pericardial structures. |
|
Subxiphoid |
The needle insertion site is between the xiphisternum and left costal margin. Once beneath the cartilage cage, lower the needle to a 15-to-30-degree angle, with the abdominal wall directed towards the left shoulder. |
A steeper angle may enter the peritoneal cavity, and a medial direction increases the risk of right atrial puncture. In some cases, the left liver lobe may be transversed intentionally if an alternative site is not available. The path to reach the fluid is longer. |
Lower risk of pneumothorax.
|
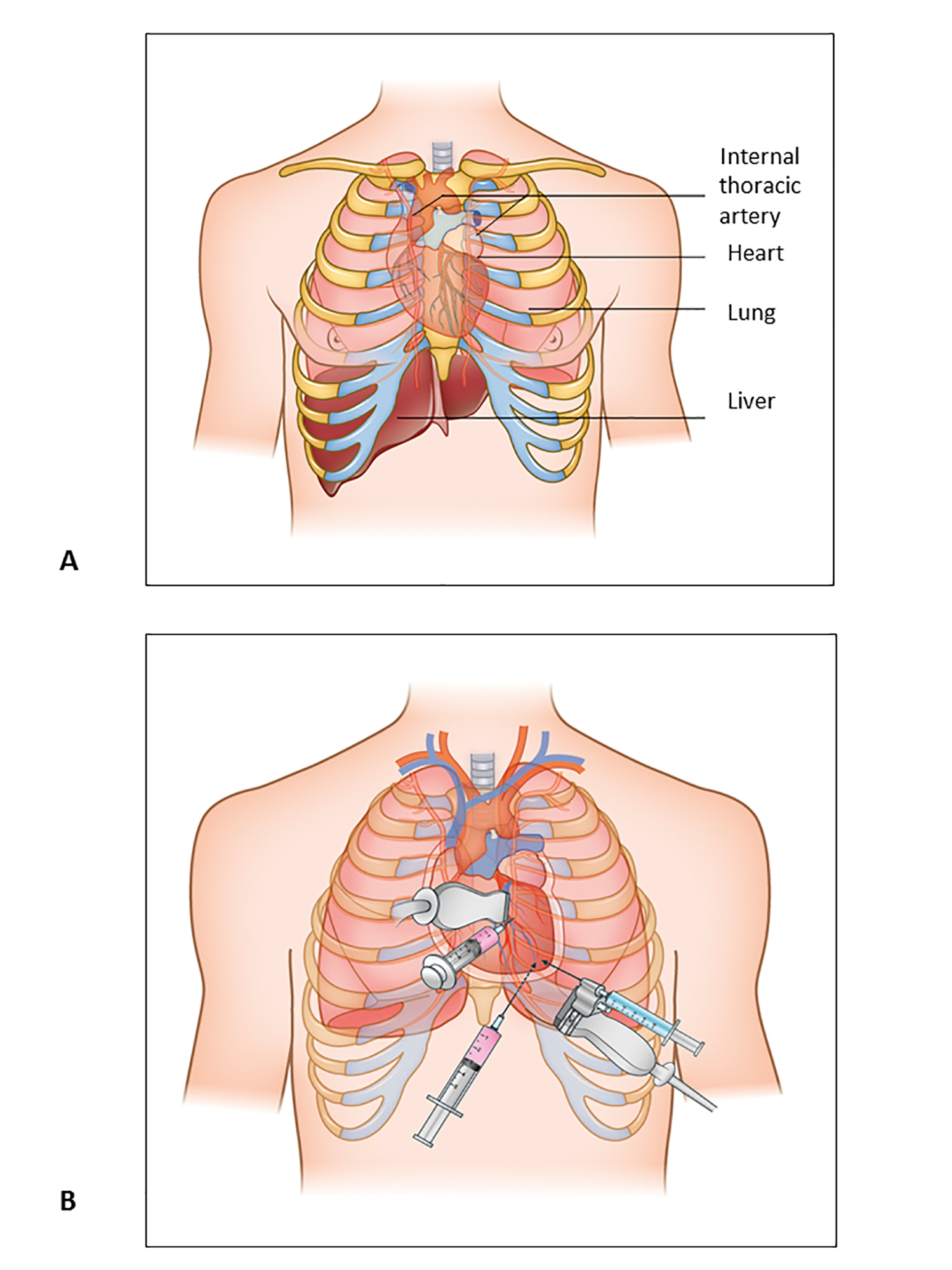
Figure 1. Pericardiocentesis: Anatomic Structures and Approaches.
A) Anatomic structures to bear in mind during pericardiocentesis procedure.
B) Three main approaches for pericardiocentesis, parasternal, substernal and apical.
Technical aspects of echo-guided procedure
An equipment list is provided in Table 2.
Table 2. Equipment Required To Perform Echo-Guided Pericardiocentesis with or without Probe-Mounted Needle System.
|
Equipment for Echo-Guided Pericardiocentesis |
|---|
|
Echocardiography with a cardiac probe |
|
Sterile probe cover and sterile echo gel |
|
16-18 gauge, Teflon-sheathed needle |
|
6 Fr to 8 Fr dilator and introducer sheath |
|
J-tipped guidewire |
|
Drainage catheter: pigtail angiocatheter 6 Fr to 8 Fr or specific pericardial drainage set |
|
Disposable flushing system to maintain patency of the catheter |
|
Equipment for Real-Time Echo-Monitored Procedure |
|
Multi-angle bracket to be mounted on the echo probe |
|
Needle guide sterile kit |
|
18 gauge, 9 cm needle (or 15 cm for subxiphoid approach) |
|
6 Fr to 8 Fr dilator |
Prior preparation is essential for the safe performance of pericardiocentesis. The platelet count and coagulation profile should be checked. Packed red cell units should be readily available before starting non-emergency procedures. Patient electrocardiographic monitoring is required in an appropriate environment with resuscitation equipment. A central venous catheter is essential for monitoring right atrial pressure and permitting rapid infusion of fluids and drugs if indicated. Continuous arterial pressure monitoring is indicated to detect the presence of pulsus paradoxus and to detect and rapidly correct sudden haemodynamic instability.
A preliminary echocardiographic evaluation is recommended with different views to assess the size and distribution of the effusion, to select the proper entry site and also to monitor the procedure. The patient should be placed in a semi-reclining position at an angle of about 30° and slightly rotated leftwards to enhance fluid collection in the inferior-anterior part of the chest. After appropriate disinfection of the operative field, a local anaesthetic is administered at the puncture site. The trajectory of the needle is defined by the angle between the probe and the chest wall. The optimal needle trajectory should be visualised in the operator’s mind, and then a 16-18 gauge, Teflon-sheathed needle with an attached saline-filled syringe advanced in the direction of the fluid-filled space. When fluid is aspirated, the needle should be advanced approximately 2 mm further. The sheath should be advanced over the needle and the steel core withdrawn, maintaining only the sheath in the pericardial space. A guidewire should be advanced through the sheath, which can then be removed. A bloody aspirate may indicate myocardial puncture or haemorrhagic pericardial effusion. The extracardiac position of the tip can be confirmed by injecting 5 ml of agitated saline infusion: the bubbles can be visualised through echocardiography in the pericardial space. A small incision should be made at the entry site followed by the introduction of a sheathed dilator (6 Fr to 8 Fr) over the guide. The dilator should be removed and a pigtail catheter inserted directly into the sheath. The pericardial effusion is aspirated by syringe suction and the catheter is closed after flushing with 5 ml of heparinised saline.
A different approach utilises a needle carrier mounted on the transducer to advance the needle under continuous visualisation (real-time, echo-monitored procedure). In most cases, the procedure is carried out by two physicians – one who performs the echocardiogram and another who performs the puncture and drainage – Figure 2.
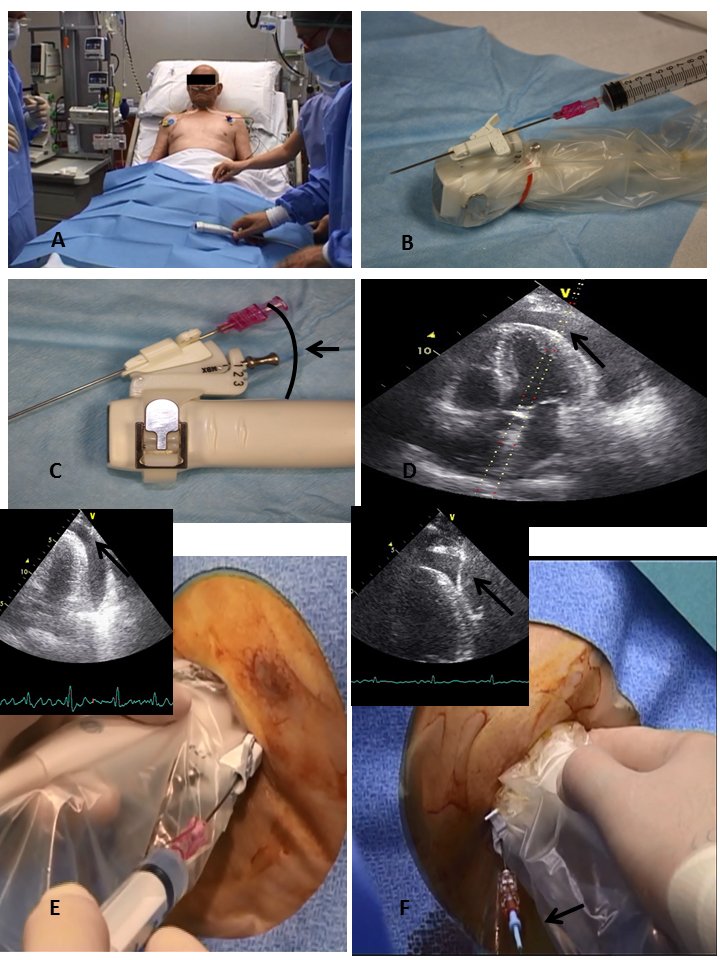
Figure 2. Echo-Guided Pericardiocentesis Procedure.
Place the patient in a semi-reclining position (Panel A), use a probe-mounted needle covered with a sterile sheath (Panel B), choose the proper angle for the needle (Panels C and D), advance the needle slowly in aspiration through the tissue until there is a continuous visualisation of the tip (black arrow) (Panel E), introduce a J-tipped wire into the needle under continuous visualisation (black arrow) (Panel F).
Patient preparation is the same as described above. The bracket should be mounted on the probe to support the needle-guide kit. The bracket supports the needle with different angles and the operator can choose between a closer or a wider angle. The probe has to be covered with a sterile sheath and the needle-guide kit has to be mounted on the sheathed probe.
Once the placement and direction of the needle are chosen, a 16-18 gauge, 9 cm needle is connected to a syringe and is slowly advanced in aspiration through the tissue until there is a continuous visualisation of the tip. When the pericardial effusion is reached and the placement is echocardiographically confirmed, a J-tipped wire is introduced into the pericardial space under continuous visualisation and a pigtail catheter should be inserted according to the Seldinger technique.
Post-procedure management
Aspiration is repeated every four to six hours, and the catheter can be removed once the drainage has decreased to less than 25 to 30 ml in 24 hours. Pericardial catheter care is the same as central venous catheter care. After the procedure, all patients undergo chest radiography to exclude the presence of pneumothorax.
Medical management
Medical management is only a temporary measure for tamponade patients while waiting for pericardiocentesis.
Hypotensive patients (systolic arterial pressure <100 mmHg) with hypovolaemia can be treated with a low volume (250-500 ml) of normal saline as it has been demonstrated to improve haemodynamic parameters. However, the infusion of higher volumes may increase wedge pressure and intrapericardial pressure, and reduce cardiac output. [13] Intravenous administration of diuretics is contraindicated and could be fatal in patients on the edge of their compensatory mechanism in tamponade. Both dopamine and dobutamine improve haemodynamics: dobutamine has greater beta activity and, therefore, it may be preferable. However, the usefulness of inotropes is generally limited because endogenous adrenergic stimulation is already enhanced under tamponade conditions. Antibiotic prophylaxis is not indicated unless the procedure has been carried out in an emergency setting without adequate asepsis. [5]
Complications
The rate of major complications reported in large observational studies for echo-guided or fluoroscopic pericardiocentesis is 0.3-3.9%, and the rate of minor complications is 0.4-20%. [5,14,15]
The most serious complications include death, injury of the cardiac chambers, laceration of the coronary arteries or intercostal vessels, puncture of the abdominal viscera or peritoneal cavity, pneumothorax requiring chest tube placement, pneumopericardium, ventricular arrhythmias and pericardial decompression syndrome. Myocardial and coronary puncture may initially be silent and present with delayed haemopericardium or intrapericardial thrombus.
Pericardial decompression is a rare, potentially life-threatening syndrome characterised by wide clinical scenarios (from pulmonary oedema to cardiogenic shock). It generally develops after a successful pericardial drainage, from a few hours to days later. The mechanism of this situation is not yet well understood. However, the simplest explanation is an acute left ventricular overload due to an increased right-sided preload associated with a persistent catecholaminergic peripheral vasoconstriction. To date, there are no effective recommendations to prevent this syndrome except to remove enough fluid to normalise the central venous and systemic blood pressure (not >1 L) and to complete the removal in the subsequent few hours. [6]
Minor complications include transient vasovagal hypotension and bradycardia, supraventricular arrhythmias, pneumothorax without haemodynamic instability, and pleuropericardial fistulas.
Several authors have suggested that particular attention must be paid to periprocedural management of anticoagulant treatment and propose that in the presence of severe coagulation disorders pericardiocentesis should be postponed until sufficient blood transfusion or other appropriate haematologic treatment is provided if overt tamponade is not present. [6] In the setting of iatrogenic pericardial effusion, full anticoagulation is considered as a major risk factor for both tamponade and its sequelae.
Pearls and pitfalls
Respiratory management
Spontaneous versus mechanical ventilation and PaCO2 levels significantly influence the evolution of pericardial tamponade. Pericardial pressure decreases 3-6 mmHg when PaCO2 decreases to 24 mmHg; conversely, pericardial pressures increase 2-4 mmHg when PaCO2 reaches 57 mmHg. Increased intrathoracic pressures during the inspiratory phase of mechanical ventilation can decrease cardiac output by up to 25% in patients with tamponade. Patients with suspected cardiac tamponade, therefore, should not receive positive-pressure ventilation unless absolutely necessary in order to avoid further haemodynamic compromise.
Catheter drainage care
The occlusion of the catheter can occur in up to 10% of cases. In order to optimise catheter patency, it could be useful to perform intermittent aspiration every six hours and use a disposable continuous flushing system between aspirations. A long period of catheter patency and limited manipulation to the effusion withdrawal are allowed, thereby reducing the chances of pericardial fluid contamination. [5]
Prevention of cardiac tamponade
Pericardial drainage for 24 to 72 hours is sufficient to avoid recurrence of pericardial tamponade in the majority of cases. The recurrence rate after the initial procedure is 27-55% for patients who undergo simple pericardiocentesis, and 12-24% for those who have extended drainage. [13,17] The omission of extended catheter drainage is an important independent predictor of recurrence. It is important to empty the pericardial sac as completely as possible, leaving the catheter in place up to 72 hours or more if the fluid has a rate of accumulation greater than 30 mL in 24 hours. Complications associated with the use of a pericardial catheter are rare. Only one case of bacteraemia has been reported in 781 cases in which this method has been used. [3,5] Re-accumulation of pericardial fluid is common in patients with malignant pericardial effusions. In these patients, several procedures have been suggested to prevent recurrence of tamponade. These approaches include repeat pericardiocentesis, which is probably the procedure of choice in patients with end-stage disease, intrapericardial sclerosis, systemic chemotherapy, radiation therapy, surgical intervention, or percutaneous balloon pericardiotomy. [18]
False negative and false positive echocardiographic findings
The most common false positive echocardiographic determinant of tamponade is right atrial collapse. This sign, as well as right ventricular diastolic collapse, can be highlighted in volume-depleted patients. In this setting, the intrapericardial pressure is at least equal to the right atrial and right ventricular pressures, which are decreased as the result of low preload. Moreover, a large pleural effusion may cause an increased intrapericardial pressure, which is sufficient to cause echocardiographic findings of cardiac tamponade. In this situation, the appropriate therapeutic approach is the drainage of the pleural effusion. [19] On the other hand, in patients with high right-sided pressures attributable to pulmonary hypertension, pulmonary embolism or right ventricular volume overload, cardiac tamponade will not demonstrate right atrial or right ventricular collapse. The reason for this phenomenon is an elevated intrapericardial pressure that does not equalise the right ventricular or right atrial pressure.
Haemorrhagic effusion
False negative results from pericardiocentesis are obtained in 20-40% of cases of haemorrhagic effusion, even when pericardial puncture can be performed without delay. [20] False negative results can be caused by the rapid formation of clots, which impede aspiration of blood. Therefore, failure to aspirate blood in cases of traumatic chest injury should not exclude the possible diagnosis of haemorrhagic effusion, delaying evacuation. In these cases, the visualisation of the needle within the pericardial space allows confirmation of reaching the space, avoiding repeated punctures.
Effects on prognosis
Pericardiocentesis is a life-saving manoeuvre when cardiac tamponade with severe haemodynamic impairment occurs and must be performed with urgency. There are no randomised studies in this setting. Once pericardiocentesis has been performed, the prognosis depends on the underlying disease, being poor in case of neoplastic aetiology and excellent in case of idiopathic/viral pericarditis. In patients with pericardial effusion without tamponade but suspected of tuberculous, bacterial or neoplastic pericarditis, pericardiocentesis is mandatory because a correct diagnosis through pericardial fluid analysis allows proper therapy and reduces the probability of an evolution towards constrictive pericarditis. In the case of chronic large pericardial effusion, the prognosis is generally good, but there may be a 35% risk of cardiac tamponade evolution. [1]
Conclusion
Pericardiocentesis can be a potentially life-saving procedure that carries a high risk of complications. In this regard, imaging support and the careful planning of the proper entry site are fundamental for a safe and successful procedure.



 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.