Electrocardiogram exercise testing
Because of its simplicity and widespread availability, treadmill or bicycle exercise ECG testing remains a useful option in patients with a low pre-test probability of coronary artery disease (CAD). Stress testing is usually performed using a treadmill or bicycle, controlled to accommodate a variety of speeds and inclines. Treadmills should be capable of providing measured increases in the speed and gradient at periods throughout the protocol. Cycle ergometers should be of a variety that can quantify the external workload in watts. Cycle ergometers for which the workload cannot be varied or where the variable workload cannot be quantified are not adequate for clinical purposes, and non-motorised or non-calibrated treadmills are similarly unsuitable. Masters two-step or other simple step devices are not considered adequate for clinical exercise stress testing, nor is any other form of non-quantified and unmonitored exercise [1,2].
Clinical utility and limitations
The main diagnostic ECG abnormality during ECG exercise testing consists of a horizontal or down-sloping ST-segment depression ≥0.1 mV, persisting for at least 0.06-0.08 s after the J-point, in one or more ECG leads. It is worth noting that, in about 15% of patients, diagnostic ST-segment changes appear only during the recovery phase [3]. For this reason, monitoring the ECG for at least five minutes after the end of exercise is strongly encouraged (Figure 1).
Figure 1. Typical ischaemic ECG changes during stress test.
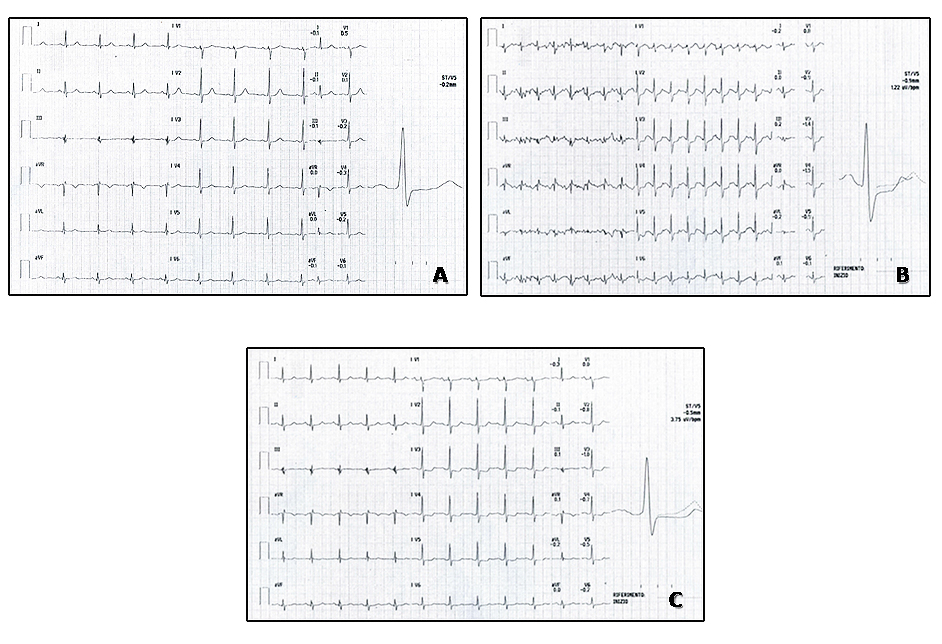
A) ECG stress testing tracing during pre-exercise phase (treadmill Bruce protocol).
B) ECG stress testing tracing, recorded at peak exercise, showing ST-segment depression in anterior leads.
C) ECG tracing during recovery phase (five minutes post-exercise), showing down-sloping ST segment in anterior leads. Subsequent coronary angiography in this patient showed a critical stenosis at the mid segment of the right coronary artery, despite the fact that ECG ischaemic alterations were only evident on anterior leads; therefore, localisation of the culprit vessel by stress ECG analysis is not reliable and should not be attempted. The development of worse ST-segment alterations during the late recovery phase confirms the indication of longer ECG monitoring in the post-exercise period.
To obtain maximal diagnostic information, exercise ECG testing should be symptom-/sign-limited. When using exercise ST depression ≥0.1 mV or 1 mm to define a positive test, the reported sensitivities and specificities for the detection of significant CAD (usually coronary artery diameter stenosis ≥50%) range between 23 and 100% (mean 68%) and 17 and 100% (mean 77%), respectively [4].
Exercise ECG testing is not of diagnostic value in the presence of left bundle branch block, paced rhythm and Wolff-Parkinson-White syndrome, in which cases the ECG changes are not interpretable. Additionally, false positive results are more frequent in patients with abnormal resting ECG in the presence of left ventricular hypertrophy, electrolyte imbalance, atrial fibrillation and use of digitalis. Exercise ECG testing is also less sensitive and specific in women [5]. However, a recent randomised trial, comparing an initial diagnostic strategy of exercise nuclear myocardial perfusion imaging (MPI) with standard exercise treadmill testing, in symptomatic women with suspected CAD who were able to exercise, did not show an incremental benefit of the more expensive MPI strategy on clinical outcomes [6].
In some patients, the exercise ECG may be inconclusive: for example, when 85% of maximum heart rate is not achieved in the absence of symptoms or signs of ischaemia, when exercise is limited by orthopaedic or other non-cardiac problems, or when ECG changes are equivocal. In these patients, an alternative non-invasive imaging test with pharmacologic stress should be selected. Heller et al [7] found that reaching only 70% compared with ≥85% of maximum age-predicted heart rate leads to a reduction in the incidence of stress defects from 100% to 47% and a reduction in angina from 84% to 26%. Maximal predicted heart rate is calculated with the simple formula “220-age”; however, most exercise test systems provide automatic calculation.
Exercise stress testing can also be useful to evaluate the efficacy of medical treatment or after revascularisation, or to assist prescription of exercise after control of symptoms. For these indications, exercise stress testing should be performed on treatment to evaluate control of ischaemia or effort performance. The effect of routine periodic exercise testing on patient outcomes has not been formally evaluated. However, an ECG stress test could be an easy and practical tool in the early post-infarction time (<6 months) in order to identify patients with residual myocardial viability rapidly. A previous study [8] demonstrated that, during ECG stress testing, the development of transient ST-segment elevation and pseudo-normalisation of inverted T-waves in the leads showing Q-waves represent a very good indicator of residual myocardial viability, compared to more sophisticated and expensive imaging perfusion/metabolism techniques.
Additional tools to increase diagnostic accuracy
Among many additional variables that supplement ST-segment depression, exercise capacity is the most powerful. Bourque et al [9] found that patients attaining <7 metabolic equivalents (METs) had an 18-fold higher prevalence of substantial (≥10%) left ventricular (LV) ischaemia compared with those reaching ≥10 METs. The value of exercise capacity is consistent in both those with and those without known CAD. As a consequence, high exercise workload is also a marker of decreased risk of cardiac events, including death [10].
Markers other than exercise-induced ST-segment depression have diagnostic and prognostic value, such as rapidity of recovery of ST-segment changes. Christman et al [11] found a low (2%) rate of positive imaging or findings of CAD on angiography and a 0.7% rate of a composite endpoint of cardiovascular death, non-fatal MI, or coronary revascularisation in patients with a positive exercise treadmill test but rapid ST-segment recovery.
ECG lead aVR is often neglected in exercise stress test interpretation. However, Uthamalingam et al [12] found a ≥1 mm aVR elevation during exercise ECG to be the strongest predictor of an obstructive left main or ostial left anterior descending artery stenosis with a diagnostic accuracy of 80% and 2.6-fold increase in post-test probability. A major limitation of the current aVR data is the absence of studies examining imaging findings and events in the general population not undergoing invasive angiography.
An increase in S-wave amplitude has been associated with subendocardial ischaemia, sometimes in the absence of ST-segment changes; this sign could represent a sensitive (although less specific) additional marker of myocardial ischaemia [13].
The appearance of negative U-waves in precordial leads during exercise has been shown to be a marker for anterior myocardial ischaemia and highly predictive of significant disease of the proximal portion of the left anterior descending coronary artery [14]. Also, exercise-induced prominent U-waves in precordial leads have been indicated as reciprocal changes for negative U-waves in inferoposterior ischaemia and as a specific marker of significant stenosis of the left circumflex or right coronary artery [15].
Several physiological markers during stress testing can augment the diagnostic accuracy of an exercise stress test and have prognostic importance. These include the heart rate and blood pressure responses to exercise and symptoms during testing. An impaired chronotropic response has been associated with a >2-fold increase in perfusion defects and a higher risk of CAD and cardiac events [16]. Heart rate recovery post exercise also carries significant diagnostic and prognostic power [17].
Finally, in patients with previous myocardial infarction, exercise-induced ventricular arrhythmias appear to be triggered by transient ischaemia occurring within a partially necrotic area containing large amounts of viable myocardium, as evidenced by positron emission tomography. Therefore, the occurrence of arrhythmias during exercise may represent a clue to the presence of residual viability/ischaemia within a previously infarcted area [18].
Exercise test in patients with arterial hypertension
The use of exercise stress testing for determining the probability of obstructive CAD in hypertensive patients lacks specificity. Undiagnosed hypertrophy may be present even in the absence of ECG signs, and exercise-induced diagnostic ST-segment depression may result from transmural flow redistribution due to increased left ventricular mass rather than to obstructive epicardial CAD. For these reasons, it has been suggested that the use of exercise stress testing to diagnose coronary artery disease in hypertensive patients with chest pain should be abandoned. However, considering its very high sensitivity, we believe that this simple procedure should be performed in all hypertensive patients with chest pain as a first screening test. In fact, exercise testing provides some additional information, useful for the management of the hypertensive patient and, when negative, is sufficient to reassure both the doctor and the patient. Conversely, when positive, the presence of coronary disease must be excluded with a supplementary non-invasive test. This is why a number of non-invasive investigations have been proposed, in an attempt to overcome the limited significance of a positive exercise test [19].
Role of myocardial spect
Essential practical aspects
Since exercise ECG cannot always provide good diagnostic accuracy for the detection of CAD, the adoption of nuclear imaging associated to exercise or pharmacological stress has been advocated for the last 40 years. The idea of cardiac nuclear imaging is based upon the flow-dependent and/or metabolism-dependent selective uptake of a radioactive tracer by functioning myocardial tissue, which can be detected by an external detector of radiation, usually a gamma camera. This method has been developed to evaluate myocardial perfusion and viability and is applied both at rest and after exercise or pharmacologic stress to assess inducible ischaemia due to flow-limiting coronary stenoses. In the early years (1970s), three planar scintigraphic images were obtained after i.v. injection of the radioactive isotope thallium-201. However, thallium-201 emits 80 keV X-rays which are suboptimal for scintillation camera imaging. To prevent some of thallium-201’s physical drawbacks, a number of radiopharmaceuticals labelled with technetium-99m have been developed. At present, the most widely used are methoxy-isobutyl-isonitrile (Sestamibi [Cardiolite]; Mallinckrodt, Staines, United Kingdom) and tetrofosmin (Myoview; GE Healthcare, Chicago, IL, USA). Myocardial uptake of these compounds occurs by diffusion and is independent of sodium-potassium pump activity. Considering the higher energy of gamma rays emitted by technetium-99m, the energy attenuation due to interposition of other tissues (breast, diaphragm) is far less intense than with thallium. Further technical developments that have improved the technique are the tomographic reconstruction of images, and electrocardiogram-gated imaging. Gated SPET allows the detection of better images and, apart from myocardial perfusion, it allows the measurement of ejection fraction, end-diastolic volume, end-systolic volume, wall motion, myocardial thickening, shortening and contractility. The term SPET means single photon emission tomography, indicating the utilisation of radionuclides emitting a single gamma ray, while positron emission tomography (PET) utilises positron-emitting radionuclides emitting pairs of gamma rays travelling in opposite directions and revealed by the detectors surrounding the subject.
Gamma cameras have been used for decades with thallium-doped sodium iodide as the detector material. Recently, new detector materials have been developed because of the physical limits of this kind of detector. The new clinically available material is cadmium zinc telluride. One manufacturer has placed 19 detector panels with pinhole collimators sharing a common focal point (the heart) and enabling an up to fourfold increase in sensitivity, first pass and other dynamic applications, radionuclide dose reduction, fast acquisition or improvement in statistics and acquisition of scans as fast as three minutes. Since these developments, the radiation dose to the patient has been considerably reduced.
The underlying principle of myocardial SPET is that, under conditions of stress, the diseased myocardium receives less blood flow than normal myocardium. SPET imaging performed after stress reveals the distribution of the radiopharmaceutical, and therefore the relative blood flow to the different regions of the myocardium. Comparing stress images to a further set of images obtained at rest permits diagnosis of stress-induced myocardial hypoperfusion (Figure 2).
Figure 2. Stress rest tetrofosmin myocardial SPET in a patient with suspected coronary disease.
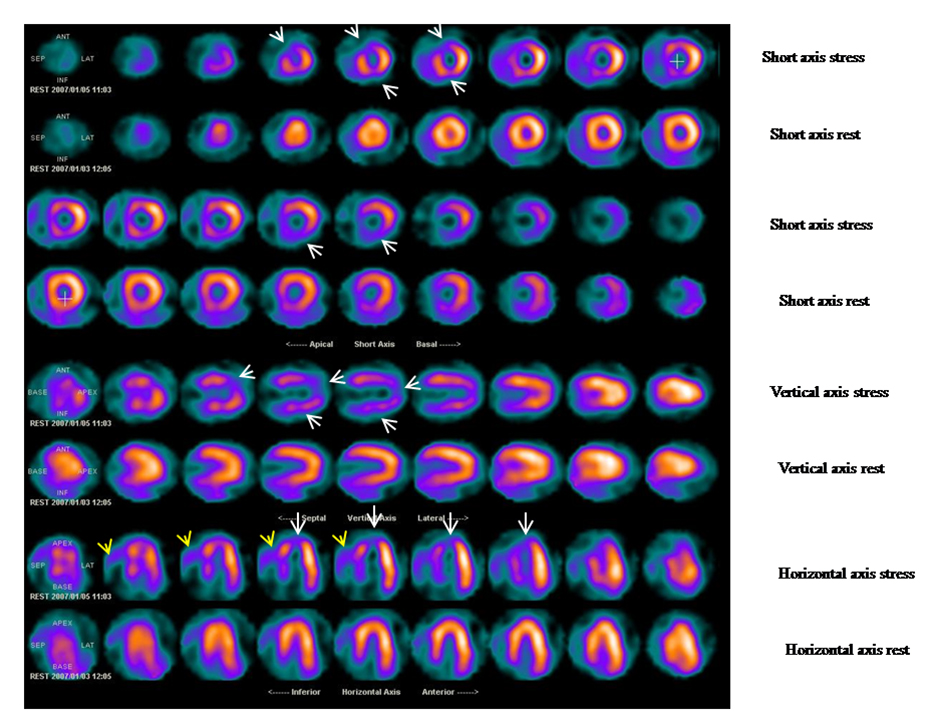
Exercise ECG results were equivocal, since the patient showed baseline significant ventricular repolarisation abnormalities. White arrows indicate a wide area of stress-induced hypoperfusion in the anterior, septal and apical segments and partly of the inferior region. Subsequent coronary angiography evidenced an ostial critical stenosis of a long left anterior descending coronary artery.
Of note, the appearance of two additional scintigraphic markers of severe inducible ischaemia. 1) There is increased right ventricular (RV) tracer uptake on stress images (yellow arrows), likely due to a global reduction in left ventricular (LV) tracer uptake at stress, resulting in a proportional increase in RV tracer uptake and/or an acute rise in RV wall stress secondary to exercise-induced ischaemic LV dysfunction, determining an increase in myocardial blood flow and radiotracer delivery to the RV. 2) Transient exercise-induced myocardial ischaemia leading to LV diastolic or systolic dysfunction determines transient left ventricular cavity dilatation. At rest, compared to stress images, a significant reduction of LV cavity dimensions is observed.
In practice, myocardial SPET is generally and preferentially performed in association with a maximal, symptom-limited exercise test. However, for patients who are unable to complete a standard exercise protocol or in the presence of right or left bundle branch block, paced rhythm and Wolff-Parkinson-White syndrome, pharmacological stress is adopted due to its better diagnostic accuracy, mainly with dipyridamole (Persantine) (at 0.56 mg/kg intravenously over a four-minute period) or adenosine (840 mcg/kg intravenously over a six-minute period). More recently, regadenoson (CVT-3146, Lexiscan; GE Healthcare), a selective A2A adenosine receptor agonist, is being preferred to adenosine, which is less selective and therefore causes more side effects. At the end of the infusion of the above-mentioned drugs and one minute after the injection of the radiopharmaceutical, intravenous administration of aminophylline (25-250 mg) is widely adopted to reverse drug-related adverse effects (chest pain, palpitation, dyspnoea, dizziness, headache, nausea, or vomiting). Patients are instructed not to ingest caffeine for 12-24 hours before the imaging examination, because caffeine can blunt the effect of commonly used pharmacologic stress induction agents.
Myocardial SPET studies are at present performed in a one-day protocol. The patient should have been fasting for at least six hours. Ongoing medical therapy should be continued/withdrawn according to the decision of the prescribing cardiologist: as a general rule, patients undergoing SPET for the first diagnosis of CAD should stop anti-ischaemic drugs, while those undergoing the test for follow-up after coronary interventions should continue their full medical therapy. Nevertheless, the final choice either to stop or to continue medical therapy before cardiac SPET studies should really be decided on an individual patient basis. Two doses of technetium-99m labelled radiopharmaceutical are required, one to be administered at peak exercise (or end of pharmacological stress) and the other for the rest study. In order to obtain a good ratio between myocardial activity and that of surrounding organs, the minimum delay from the administration of the dose to scanning the patient is 40 minutes. At present, with the most modern dedicated gamma cameras, the recommended doses for tetrofosmin imaging are 185-296 MBq (5-8 mCi) of Tc99m tetrofosmin at peak exercise and 370-592 MBq (10-16 mCi) of Tc99m tetrofosmin at rest, approximately two hours later, determining a total radiation dose to the patient of around 4.6 mSv. As for comparison, the radiation dose to a patient undergoing coronary computed tomographic angiography (CTA) varies from 1 mSv to 10 mSv; the variability gap related to specific acquisition protocols cannot however be applied to all patients. Soon after radionuclide administration, patients are administered a small fatty meal to stimulate gall-bladder emptying and decrease radionuclide liver uptake.
At present, when stress perfusion is pretty normal, the rest acquisition is usually not performed, in order to avoid the administration of the second dose of radiopharmaceutical and, as a consequence, to reduce the radiation exposure of the test. The results of several studies have confirmed that mortality rates after normal findings at stress-only perfusion imaging are similar to those after combined rest-stress perfusion imaging.
Myocardial SPET clinical applications
Many clinical cardiologists prefer to start the initial diagnostic workup of patients with suspected CAD directly with stress/rest myocardial perfusion SPET. This is of course due to the greater diagnostic accuracy of nuclear imaging, but this approach is not entirely justified by the evidence. In fact, a patient with a normal baseline ECG and absence of left ventricular hypertrophy can certainly undergo a plain ECG stress test without associated imaging, maintaining a good diagnostic accuracy. On the other hand, nuclear imaging is mandatory in all patients presenting with abnormal baseline ECG: electrical evidence of left ventricular hypertrophy, repolarisation abnormalities, intrinsic or related to ongoing therapies (one for all: digoxin), and intraventricular conduction abnormalities are all frequent conditions in the average patient. In these cases, the diagnostic accuracy of exercise ECG testing is low and therefore stress/rest myocardial perfusion SPET is advocated.
On the other hand, there are specific subsets where myocardial SPET appears necessary. In symptomatic patients with haemodynamically “non-significant” coronary stenosis, it is important to evaluate visually the real magnitude of residual ischaemia, after optimisation of medical therapy, in order to decide whether or not and, eventually, which vessel to revascularise. In patients previously revascularised, sometimes with a medical history of multiple surgical and percutaneous revascularisation procedures, it may be very difficult to decide further management only with exercise ECG and anatomic information. In these cases, complex native, collateral and graft circulation can be effectively assessed by stress perfusion imaging studies in order to choose the best therapeutic approach for the individual patient. At the end of the day, what really counts in prognosis is ischaemia, not vessel stenosis.
Significance of myocardial perfusion abnormalities in patients with epicardial coronary artery disease
The good prognostic significance of myocardial perfusion abnormalities in patients with CAD [20] appears particularly relevant in view of previous evidence suggesting that searching for ischaemia rather than the mere presence of atherosclerotic stenosis could represent a better prognostic tool even in patients with macroscopic coronary artery disease. In the COURAGE trial (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation), a strategy-driven trial randomising 2,287 patients to optimal medical therapy (OMT) with or without percutaneous coronary intervention (PCI), no difference was revealed by treatment in the primary endpoint of death or acute myocardial infarction (MI) for a median 4.6 years of follow-up (p=0.62). The COURAGE trial included a nuclear substudy to measure ischaemic burden in a subset of patients [21]. The primary aim of the nuclear substudy was to compare changes in ischaemic burden after randomisation to PCI+OMT compared with OMT alone and to explore associations with patient outcome. The nuclear substudy demonstrated that the magnitude of ischaemia on MPI was proportional to the risk for death or MI. Regardless of treatment assignment, the magnitude of residual ischaemia on follow-up myocardial SPET was proportional to the risk of death or myocardial infarction, and a ≥5% reduction in ischaemia by intervention was associated with a significant reduction in risk. Of the OMT patients exhibiting significant ischaemia reduction, the majority were also angina-free, generally with mild residual ischaemia on their follow-up MPI.
More recent evidence supporting the non-inferiority of functional compared to anatomical testing for risk stratification has been provided by the PROMISE study [22]. Ten thousand and three (10,003) symptomatic patients were randomly assigned to a strategy of initial anatomical testing with the use of CTA or to functional testing (exercise electrocardiography, nuclear stress testing, or stress echocardiography). The composite primary endpoint was death, myocardial infarction, hospitalisation for unstable angina, or major procedural complication. Secondary endpoints included invasive cardiac catheterisation that did not show obstructive CAD and radiation exposure. They found that, in symptomatic patients with suspected CAD who required non-invasive testing, a strategy of initial CTA, as compared with functional testing, did not improve clinical outcomes (over a median follow-up of two years). In fact, there was a non-significant trend towards better performance in the functional testing arm: during follow-up, 164 patients (3.3%) in the CTA group and 151 (3.0%) in the functional testing group had a primary endpoint event (p=0.75). In conclusion, based on these results, in symptomatic patients with suspected CAD who require non-invasive testing, an initial strategy of coronary anatomy assessment was not associated with better clinical outcomes than functional testing over a median follow-up of two years.
Significance of myocardial perfusion abnormalities in patients with microvascular coronary artery disease
Cardiac syndrome X is characterised by angina-like chest pain and positive exercise test, in the presence of angiographically normal coronary arteries. Although multiple pathogenetic causes have been hypothesised, coronary microvascular dysfunction appears as a likely mechanism in a sizeable proportion of patients. Some studies conducted in patients with cardiac syndrome X reported reduced progression of the angiographic dye (“slow-flow”) and suggested that this phenomenon could possibly be caused by small vessel disease. Direct evidence of transient reversible myocardial underperfusion during MPI occurring during slow-flow has been demonstrated and associated with a worse long-term prognosis [23].
More recently, the conventional stress/rest MPI has also been confirmed to be a very useful prognostic tool in patients with syndrome X [24], where the observation of stress perfusion defects had traditionally been considered as a “false positive” result. In this study, prognosis in patients with normal coronary arteries but scintigraphic evidence of relatively mild inducible myocardial hypoperfusion was not as good as in patients with normal perfusion, especially in terms of morbidity. In fact, in those syndrome x patients showing transient scintigraphic perfusion defects, a worse combined survival and hospitalisation rate, and greater and longer symptomatic burden requiring multidrug therapy were observed. More specifically, the significant increment of the secondary endpoint (cardiovascular hospitalisations) and the greater symptomatic burden in the positive MPI group clearly indicate a worse functional prognosis in these patients. On the other hand, these results also indirectly confirm the association between a negative myocardial SPET and very low event rates [25].
Artefacts and peculiar perfusion patterns
Despite the fact that scatter correction and resolution recovery processes are commonly incorporated in the reconstruction of attenuation-corrected images, the most frequent artefacts in SPET analysis are related to soft tissue attenuation of radiation detection, depending on the individual patient’s body habitus. A female breast, potentially causing artefactual anterior defects, is usually fixed with adhesive tape in order to maintain it in the same position after stress and at rest. If the breast is in the same position during stress and rest imaging, the potential apparent perfusion defect will be present on both sets of images. However, if breast attenuation significantly degrades the quality of images, image acquisition can be repeated with the patient in the prone position, determining a position change of the breast with respect to the heart and therefore decreasing the degree of soft tissue attenuation.
Due to hepatobiliary excretion of technetium 99m-labelled radiotracers, prominent sub-diaphragmatic activity can be observed in the liver and bowel and also, occasionally, in the stomach. This radioactivity can affect myocardial SPET imaging, principally because scatter radiation from the radiotracer can lead to an apparently increased perfusion in the inferior myocardial wall that might mask a true perfusion defect in the same region. This event is observed mainly when patients after radionuclide administration miss the fatty meal, which stimulates gall-bladder emptying and decreases radionuclide liver uptake.
Undue patient motion
Particular attention must be paid to patient motion during the acquisition of images: in fact, patient motion, often related to respiration, is the most common source of artefacts on myocardial SPET.
Apical thinning
Apical thinning is a normal anatomic finding. The aetiology of this finding is multifactorial. In some people, thinning may be more prominent than usual and may simulate a perfusion defect. Apical thinning is more apparent on attenuation-corrected images and may be accentuated by the scatter correction.
Left main or three-vessel CAD
Maximum-stress SPET may be normal in some patients with a global reduction in myocardial perfusion and show no focal perfusion defect despite the presence of left main or three-vessel CAD. This results in the underestimation or missed diagnosis of ischaemia in patients with balanced coronary ischaemia. In fact, some studies have suggested that SPET alone has a low sensitivity in detecting left main coronary disease. In order to overcome this limitation, in addition to myocardial perfusion defects which reflect functional ischaemia, there are ancillary findings that, if present, independently predict a higher risk of CAD and future cardiac events. These are especially important in cases where the perfusion may appear “normal”. In patients with CAD, exercise-induced myocardial ischaemia leads to LV diastolic or systolic dysfunction and determines an elevated LV end-diastolic pressure. The consequent increased pulmonary capillary pressure with increased leakage of radiotracer into interstitial spaces will eventually result in increased pulmonary radiotracer uptake, which can be detected by the expert SPET interpreter. The detection of transient exercise-induced ischaemic left ventricular cavity dilatation may also represent a useful sign of significant CAD when interpreting myocardial SPET studies. Finally, increased right ventricle tracer uptake on stress images could also be a marker of severe CAD. In fact, a global reduction in LV tracer uptake at stress, resulting in a proportional increase in right ventricular (RV) tracer uptake and/or an acute rise in RV wall stress secondary to exercise-induced ischaemic LV dysfunction, could end up in an increase in myocardial blood flow and radiotracer delivery to the RV. These ancillary signs have been indicated as a useful tool to identify patients with severe CAD and balanced ischaemia and should therefore be systematically considered when analysing a myocardial SPET study. Therefore, when dealing with individual patients, the referring cardiologist and the interpreter of SPET studies should consider the presence of severe CAD when there are markers of CAD despite normal or near normal perfusion images.
The "reverse redistribution" phenomenon
The "reverse redistribution" phenomenon refers to a myocardial perfusion defect that develops on rest imaging, whereas scans acquired after stress show an apparently uniform distribution. This finding has been observed with thallium-201 in a variety of cardiac conditions [26]. Technetium-99m-labelled radio-pharmaceuticals may also yield a “reverse perfusion” pattern. As for thallium, some authors consider reverse perfusion of Tc-99m-labelled tracers a mere artefact, without clinical significance. Conversely, this phenomenon has been associated by others to coronary artery disease, previous myocardial infarction with and without coronary artery disease [27]. Nevertheless, as underlined above, in order to reduce patients’ radiation exposure, rest imaging is not performed anymore in the presence of a normal stress scan and, therefore, this peculiar perfusion pattern is nowadays less frequently observed.
Clinical implications and conclusions
In patients with suspected or established coronary disease, physiological testing remains the gold standard, especially in the prognostic evaluation of the disease. In this context, exercise test testing and, when indicated, myocardial perfusion scintigraphy appear to be very accurate techniques [28]. Indeed, as is evident in patients with either epicardial or microvascular coronary artery disease, the presence and extent of inducible myocardial ischaemia grossly correlate with clinical outcome and should therefore be considered as the most relevant factors for performing a close follow-up of these patients. In fact, anatomic evaluation of obstructive stenoses does not determine the haemodynamic significance of the visualised lesions.
In conclusion, functional testing remains the most useful tool for risk stratification in macrovascular and microvascular coronary disease. A combined anatomic-physiological approach, using recent technical advances, could possibly become the best diagnostic/prognostic tool in the very near future.




 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.