Introduction
Right heart failure (RHF) syndrome is characterised by the inability of the right ventricle to generate enough stroke volume, thereby resulting in systemic venous congestion, underfilling of the left ventricle and, in the most advanced cases, cardiogenic shock. Right heart failure portends a poor prognosis in almost every clinical scenario [1-3]. Although the aetiologies of right ventricular (RV) failure are diverse, treatment often involves simultaneous and timely execution of multiple strategies aimed at optimising RV preload, afterload, and contractility. Amelioration of the primary driver of RV failure and reducing further RV insult when feasible are desirable. Timely institution of mechanical circulatory support can offer a bridge to RV recovery or to definitive management of the underlying cause (Figure 1).
Figure 1. Principles of managing right heart failure.
Although the general principles of managing RHF apply to both the acute and chronic settings, the following discussion highlights the nuances and challenges of managing isolated RHF in the acute setting (Figure 2). Management of the specific causes of right heart failure, such as pulmonary hypertension, pulmonary embolism, RV infarction, left ventricular dysfunction, sepsis etc., is beyond the scope of this article.
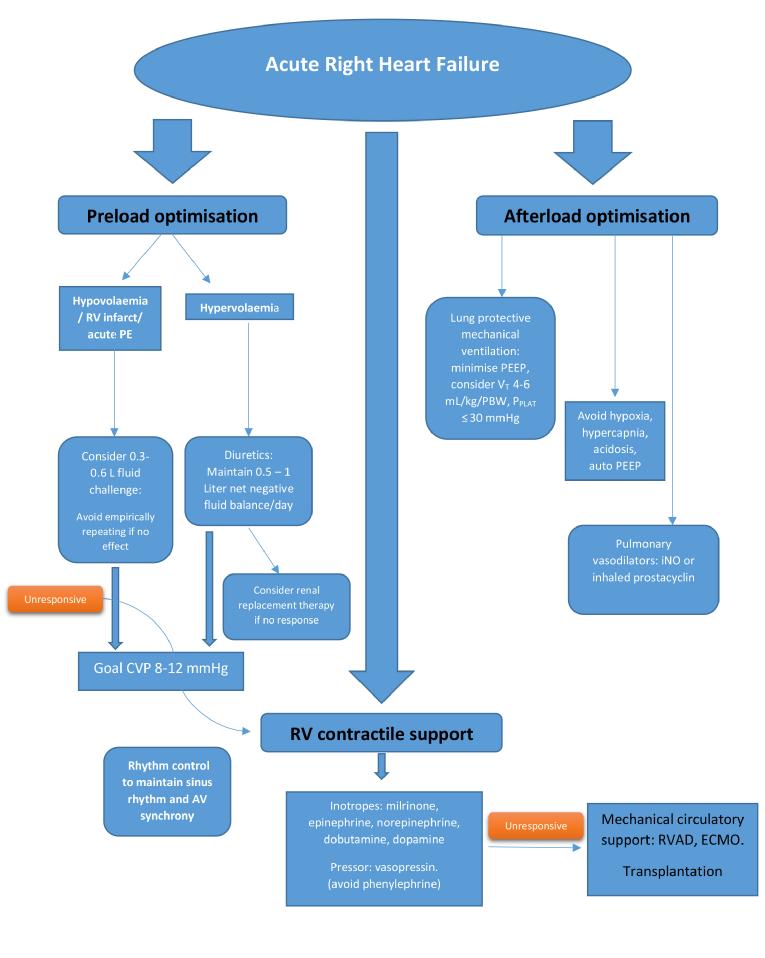
Figure 2. Management of isolated acute right heart failure.
Modified from Hadad et al [23] and Lahm et al [24].
CVP: central venous pressure; ECMO: extracorporeal membrane oxygenation; iNO: inhaled nitric oxide; PE: pulmonary embolism; PEEP: positive end-expiratory pressure; PPLAT: plateau pressure; RV: right ventricle; RVAD: right ventricular assist device; VT: tidal volume
1. Optimisation of RV preload, afterload and contractility
(a) Preload optimisation
Critically ill patients may have reduced right heart preload due to volume loss, reduced venous tone from medications, sepsis or vasoplegia, and positive pressure ventilation. Although the Frank-Starling mechanism is preserved in the failing human RV myocardium [4], the right ventricle has a much flatter Frank-Starling curve than the left ventricle, and therefore RV contractility changes less over a wide range of filling pressures. In conditions where RV output is impaired due to contractile dysfunction but the afterload is normal (as can be seen in acute RV infarction), a higher preload is needed to maintain forward flow. However, the majority of conditions leading to RHF are characterised by high RV afterload. In these scenarios, reducing excessive RV preload with diuretics or haemofiltration is key to reducing RV dilatation and free wall tension, thereby minimising RV ischaemia and optimising contractility. It is generally agreed that maintaining a moderately high RV diastolic filling pressure of 8-12 mmHg is optimal in RHF. However, the goal of maintaining forward flow may be achievable at more normal filling pressures in individual cases.
(b) Afterload reduction
Strategies for optimising RV afterload can be divided into general measures and pharmacotherapy, including pulmonary vasodilators. General measures are aimed at correcting conditions that can increase pulmonary vascular resistance (PVR) in critically ill patients. These conditions include acidosis, hypoxia (which causes pulmonary vasoconstriction), and hypercapnia. Lung protective ventilation, using the lowest effective plateau pressure, tidal volume, and positive end-expiratory pressure while avoiding hypoxaemia and hypercarbia, assists with optimising both RV preload and afterload [5].
Pulmonary vasodilators include several classes of drug that are approved for use in pulmonary arterial hypertension (PAH). However, vasodilators have the potential to cause systemic hypotension. Pulmonary vasodilators can also blunt hypoxic pulmonary vasoconstriction and thereby worsen ventilation perfusion matching. Pulmonary vasodilators are currently not approved for use in critically ill patients with RV failure not due to PAH. However, inhaled nitric oxide (iNO), used off-label, with its rapid onset of action, short half-life and inhaled route of administration, is the pulmonary vasodilator of choice in the critically ill and has been shown to improve pulmonary haemodynamics in RHF [6]. Inhaled prostacyclin analogues have been shown to be safe and effective pulmonary vasodilators for cardiothoracic surgical patients with pulmonary hypertension, refractory hypoxaemia, or right heart dysfunction, and they may offer substantial cost savings over iNO [7]. Phosphodiesterase 5 (PDE5) inhibitors block degradation of cyclic guanosine monophosphate. They decrease pulmonary arterial pressure and increase cardiac output in both acute and chronic pulmonary hypertension. PDE5 inhibitors have also been shown to increase RV contractility in an animal model of hypertrophied right ventricles [8]. Combined with their ability to reduce afterload, PDE5 inhibitors are sometimes used in patients with acute RHF and underlying chronic pulmonary hypertension. One such example is the off-label use of PDE5 inhibitors in the management of RHF after left ventricular assist device implantation [9]. PDE5 inhibitors are contraindicated in patients on nitrates and should be used with caution in haemodynamically unstable patients.
(c) Improving contractility
Strategies to improve RV contractility can also be divided into general and pharmacologic measures. General measures target the avoidance of overstretching the RV free wall by optimising preload and afterload - maintaining RV perfusion by reducing wall tension and increasing coronary artery diastolic pressure and maintaining a cellular milieu conducive to myocyte contractility. Maintenance of sinus rhythm is important in RHF, as supraventricular tachyarrythmias (SVT) can further compromise cardiac function. In a cohort of 231 patients with PAH and chronic thromboembolic pulmonary hypertension, there was an 11.7% cumulative incidence of SVT, and restoration of sinus rhythm was associated with recovery from RHF [10]. Atrial or atrioventricular sequential pacing in the setting of chronotropic incompetence may instantaneously improve cardiac output in RHF.
Concerning inotropes, in animal models low-dose dobutamine (5-10 μg/kg/min) has been shown to restore RV-pulmonary artery coupling and cardiac output better than norepinephrine because of its more pronounced inotropic effect [11]. Given that it is an inodilator, it is preferably used in normotensive patients.
Dopamine also improves RV contractility and, at doses below 16 μg/kg/min, it increases cardiac output without increasing PVR [12]. Dopamine would be preferable for hypotensive, non-tachycardia patients.
Milrinone, a selective PDE3 inhibitor, slows intracellular cyclic adenosine monophosphate (cAMP) metabolism and improves inotropy and facilitates pulmonary vasodilation. It is an attractive agent in pulmonary hypertension resulting from biventricular failure [13]. In normotensive patients on chronic beta-blocker therapy, milrinone would be preferable. Inhaled milrinone has also been investigated for management of RHF as it avoids the side effect of systemic hypotension. In a randomised trial of high-risk cardiac surgery patients, inhaled milrinone was associated with increases in cardiac output and a reduction in pulmonary artery systolic pressure without causing systemic hypotension. Nevertheless, these favourable haemodynamic effects did not translate into improvement of clinically relevant endpoints [14].
Epinephrine has been shown to improve cardiac output without detrimentally affecting PVR in an animal study [15]. Norepinephrine causes vasoconstriction via α1 receptor stimulation and has limited β1 inotropic effects. Although it may increase PVR, these effects may be offset by the improved RV-pulmonary artery coupling [16]. Both epinephrine and norepinephrine would be preferable in hypotensive patients.
Levosimendan, a calcium sensitiser, has been shown to improve RV function in left heart disease [17]. However, in a study comparing levosimendan with milrinone in cardiac surgery patients, levosimendan resulted in a greater increase in heart rate, decrease in systemic vascular resistance, and a greater need for norepinephrine [18]. Levosimendan is currently not available in the USA. Finally, digoxin therapy given acutely in pulmonary hypertension may improve cardiac output modestly [19].
The right ventricle is coupled in series with the left ventricle. As RV failure progresses, the interventricular septum bulges to the left, impairing left ventricular filling as well as reducing the septal contribution to RV contractility. This unfavourable left-sided septal shift can lead to hypotension, which reduces coronary perfusion pressure, resulting in RV ischaemia. Rising right-sided filling pressures and systemic hypotension can also promote right to left shunting in patients with intracardiac shunt, typically with patent foramen ovale, thereby exacerbating hypoxia.
In the hypotensive patient with severely elevated pulmonary artery pressure, vasopressors are often used to raise systemic pressure. Generally, the goal is to keep the systemic arterial pressure higher than pulmonary arterial pressure. An ideal pharmacologic agent to achieve this goal would raise systemic pressure without detrimentally affecting PVR. Vasopressin binds to V1 receptors on vascular smooth muscles and at low doses (0.01-0.03 U/min) causes pulmonary vasodilatation via endothelial nitric oxide stimulation. However, at higher doses vasopressin can cause coronary vasoconstriction by increasing catecholamine responsiveness [20,21]. The role of epinephrine and norepinephrine has been reviewed under inotropes. Phenylephrine, a pure α1 agonist, can increase pulmonary vascular resistance, and for that reason we tend to avoid this drug in RHF.
2. Mechanical circulatory support devices and treating the underlying cause of right heart failure
Although a detailed discussion of the management of specific aetiologies of RV failure is beyond the scope of this article, the identification and management of treatable causes of RV failure is an important aspect of management strategy. Examples include thrombolytic or catheter-based management for massive pulmonary embolism and early coronary reperfusion for RV infarction.
In cases refractory to medical therapy, timely deployment of mechanical circulatory support may offer a bridge to recovery or to the definitive management of the underlying cause. Percutaneous support options include extracorporeal membrane oxygenation (ECMO), which offers right- and left-sided circulatory support. Currently available options for percutaneous right ventricular assist devices include the Impella RP System (Abiomed, Danvers, MA, USA), and TandemLife (CardiacAssist, Inc. Pittsburgh, PA, USA) using the PROTEK Duo cannula. Surgically cannulated pumps such as the Levitronix CentriMag left ventricular assist system (Levitronix LLC, Waltham, MA, USA) can also offer RV support when employed in a right atrial and pulmonary artery configuration. Although none of the durable ventricular assist devices is currently approved for RV support, their use has been reported. [22] Select patients with refractory RHF may be candidates for transplantation.
Conclusion
The management of isolated acute right heart failure remains more of an art than a science in the absence of robust randomised data. In addition to treating the specific cause, RV preload optimisation, the use of selective pulmonary vasodilators, RV inotropic support and temporary mechanical circulatory device therapy form integral components of a comprehensive strategy to support the failing right heart.















