Introduction
The incidence of aortic stenosis (AS) is growing markedly due to the increase in the mean age of the population. In recent years, transcatheter aortic valvular implantation (TAVI) has become a valuable alternative to prosthetic surgical replacement of the valve (SAVR) [1,2]. Consequently, a large population of very old patients with many comorbidities, previously considered inoperable, have been successfully submitted to TAVI procedures. However, this very frail population has particular characteristics and needs special attention during follow-up (FU) to achieve a successful outcome and avoid early and late complications affecting patients’ survival and quality of life (QoL) [3]. In this review, we present and discuss the main issues of the FU of TAVI patients. These issues may be related to patients’ clinical problems, to TAVI-specific valve problems, to prosthetic problems common to other types of heart valve prosthesis, to management problems and, finally, to organizational problems.
Patients’ clinical problems
- Age
- Frailty
- Bleeding
- Vascular problems
- Infections
- Kidney and lung dysfunction
- Cerebrovascular problems
- Pre-existing conduction defects
- Other comorbidities
In this very old population, the existence of many comorbidities, which may deteriorate during the intervention or may influence the result of the procedure, is quite frequent. Frailty may be a consequence of age, reflecting a reduced ability of biological reactions of the body to respond to each injury, including that of the procedure itself. This concept will have been evaluated in the pre-TAVI candidate evaluation, but is relevant also during the FU. Patients identified for their frailty should be followed with specific dedicated care.
Bleeding and vascular problems are important prognostic factors which have a definite impact on post-TAVI outcome. They are directly related to the character of TAVI but are becoming less frequent with the evolution of prosthesis technology and improvement of the operators’ learning curve. Infections should be prevented and rapidly dealt with if they occur, since they may easily become fatal in this complex population.
Kidney and lung dysfunction complicates the outcome of any procedure and is directly related to morbidity and mortality during TAVI FU.
Also, pre-existing cerebrovascular events in TAVI patients predispose to an enhanced incidence of complications during the short- and long-term period after TAVI.
Conduction defects may predispose and develop to complete AV blocks requiring a pacemaker (PM) implant. They may be inherent in the nature of AS itself, but may also be TAVI-induced (see below). Their evolution is often unpredictable and requires careful monitoring during FU.
Finally, despite being underevaluated, depression, as well as other psychiatric disorders with a strong impact on the outcome of every cardiovascular disease, should always be carefully evaluated.
Valve problems
Any FU program for TAVI patients should include imaging, particularly echocardiography, as a main step. As in the pre-TAVI evaluation, ejection fraction (EF), regional kinesis, pulmonary artery pressure (PAP) and left ventricle (LV) hypertrophy should be assessed, and other valve dysfunction should also be detected and followed up [4].
The cardiologists should focus on the possible common prosthetic complications, which may also affect the TAVI prosthesis, and, additionally, should know and recognize the specific TAVI prosthesis problems [5,6].
Prosthetic problems
Infective Endocarditis (IE)
The incidence of TAVI IE is about 1.1% [7], with a median time of manifestation of six months; 18% of cases occur early (<60 days), 62% in an intermediate time (60 days-1 year) and 20% during late FU (>1 year) [5,6].
Risk factors for TAVI IE may be the common factors as for IE on native valves or SAVR valves: diabetes, chronic kidney disease, immunosuppression, oral hygiene, and recurrent infections. Specific risk factors for TAVI prostheses are: a non-sterile environment of cathlabs (which may argue in favor of hybrid operating theatres for these procedures), suboptimal valve positioning and injury to the anterior mitral leaflet, failure to administer antibiotic prophylaxis before TAVI, and dental procedures. In particular, the latter are often overlooked.
The diagnosis might be challenging in old patients, since symptoms are insidious and atypical (fever, rigors, progressive malaise, progressive or acute heart failure, peripheral embolism with arm and leg ischemia, or stroke), and blood culture tests may be positive (79% of 34 cases) or not. Echocardiographic findings may include: mobile vegetations, abscess formation, progressive stenosis or, more frequently, prosthesis regurgitation (positivity 86%). Therapy is based on targeted antibiotics and surgical management which, considering the very dangerous condition, demonstrated a fairly good outcome (38-75% survival) [6,7].
Thrombosis
Thrombosis of a TAVI prosthesis is very rare (up to 0.8%) and occurs, mainly in Edwards SAPIEN prostheses (Edwards Lifesciences, Irvine, CA, USA), at a mean time of 9±7 months (1-24 months) after the implant [6]. Risk factors are: coexisting prothrombotic conditions (such as cancer), incomplete expansion and/or apposition to the aortic wall, and native leaflets overhanging the balloon-expandable systems. Symptoms consist mainly of a progressive dyspnea, but they have also been shown to include NSTEMI, heart failure and cardiac arrest. Echocardiographic findings are: increased transvalvular gradients, leaflet thickening and direct visualization of thrombotic formations. Differential diagnosis is with IE. The treatment of choice is intensive oral anticoagulation which can, in a relatively short time, lead to the normalization of gradients and leaflet mobility.
Structural failure
Structural failure of TAVI valves is very rare (13 reported cases: nine Edwards SAPIEN and four CoreValve) [6]. It occurs at 24±26 months, with a range from a few days to 5.5 years. It has been related to incomplete frame expansion, elliptically deployed prostheses and anomalies in the pre-crimping process. These events may lead to leaflet calcification with severe AS or to cusp rupture with severe AR; sometimes tissue ingrowth (pannus) has been shown. Possible symptoms are dyspnea or heart failure though some patients are quite asymptomatic. Echocardiographic findings are moderate or severe AS/AR. A differential diagnosis from thrombosis may sometimes be difficult and multimodality imaging is needed. The treatment of choice is TAVI-in-TAVI.
Patient-prosthesis mismatch (PPM)
This occurs when the effective orifice area of the prosthetic valve is too small in relation to the patient's body size and is associated with worse outcome. This will hopefully be a very unusual occurrence since current TAVI prosthesis sizing relies upon multimodality imaging. A post hoc analysis of the PARTNER cohort A trial showed a higher incidence of PPM in SAVR than in TAVI (28% vs. 20%), with a more pronounced difference when dealing with small aortic annulus diameters (<20 mm) (36% vs. 19%) [8].
TAVI-specific issues
Aortic regurgitation (AR)
This is mainly paravalvular, and is one of the most important issues to consider during the FU of TAVI. In particular, paravalvular aortic regurgitation (PAR), due to incomplete apposition of the prosthesis with the aortic annulus, represents the main current limitation of the TAVI procedure. The incidence of moderate to severe AR could reach 11.7% of patients; it increases the risk of all-cause mortality and morbidity. The degree of AR may remain stable over time, or may worsen and deteriorate, but it may also decrease at one- and two-year FU.
Some attempts at standardization of the methodology and timings to evaluate AR at FU will help to elucidate the time course of AR after TAVI and its prognostic implications [9]. The echocardiographic assessment of AR grade after TAVI is based on the Valve Academic Research Consortium (VARC) criteria [10]. The main parameters to consider are:
- Regurgitant jet width relative to LV outflow tract diameter (transthoracic echo parasternal long-axis view or transesophageal echo 120°-140° view). This parameter allows a semi-quantitative assessment of transvalvular AR: grading ≤25%, 26-64%, ≥65% defines mild, moderate, or severe AR, respectively.
- For PAR, eccentric or multiple jets: the proportion of the circumference of the prosthesis covered by the AR jet in the short-axis view. Mild, moderate, and severe PAR are defined as <10%, 10-29% and ≥30% extent of the circumference of the prosthesis frame.
Three-dimensional transesophageal echo allows direct visualization and planimetry of the vena contracta [11]. Velocity-encoded magnetic resonance imaging (MRI), which allows the direct measurement of blood flow velocity and volume across the valve and calculation of the regurgitant fraction, has gained increasing interest [12].
Conduction defects
The mechanical interaction of the prosthesis stent frame with the conduction system, located at the atrioventricular (AV) junction just proximal to the aortic valve plane, and with the left bundle branch may lead to a high degree of or complete AV block and to left bundle branch block (LBBB) after TAVI. A pre-existing conduction defect, male gender and age have been demonstrated to be predictors of high-degree blocks in TAVI patients. These complications may appear with the balloon-expandable Edwards SAPIEN in 16-28% of patients. In more than 50% they generally disappear in a few days, while ~6% (4-13%) of patients need a permanent PM. High-degree conduction defects are more frequent with the self-expanding CoreValve (Medtronic, Minneapolis, MN, USA) system (38-57%). They mostly persist at hospital discharge and one-year FU; ~25% (11-39%) of patients require a PM.
Continuous ECG monitoring should be undertaken for at least 48 hours in every patient who develops a new LBBB before discharge. The indications for long-term FU are less defined: an ECG is mandatory at every visit during the FU program. ECG Holter monitoring is also reasonable, but the timing of these controls is not clear. New-onset LBBB and PM implantation, including its protective or detrimental effect, seem to be independent predictors of outcome, but their significance is currently unassessed.
Late thromboembolism (stroke) and prophylaxis
Ischemic stroke may occur during or after TAVI, either in the first days or during long-term FU. In the PARTNER trial, the incidence of stroke at 30 days was higher in TAVI patients as compared with medical and surgical ones: TAVI vs. medical therapy 6.7% vs. 1.7% (p=0.03); TAVI vs. SAVR in 5.5% vs. 2.4% (p=0.04). In two meta-analyses comparing TAVI vs. SAVR, stroke rates were 3.5% vs. 2.8% and 2.6% vs. 2.3%.
Actually, most strokes occur within 30 days from the intervention with a peak at 48 hours. However, one in four are late events, after 30 days and sometimes also after many months. These subacute and late episodes are mainly of thromboembolic origin, which could arise from the stent of the implanted valves, but more frequently from atrial fibrillation (AF) occurrence. A high aortic atherosclerotic burden, a previous cerebrovascular event (CVE), peripheral vascular disease and permanent AF have been identified as the main predictors of late CVEs after TAVI.
Pharmacological measures, such as an intensified antiplatelet therapy and/or a more aggressive anticoagulation regimen during and after TAVI, have been proposed for the prevention of this complication.
The current standard of care for TAVI patients is double antiplatelet therapy with low-dose aspirin (75-100 mg) and clopidogrel 75 mg without any specification as to loading dose and duration of therapy. Besides, the benefit of clopidogrel is questioned in elderly patients for several reasons: 1) high on-clopidogrel platelet reactivity, 2) risk of bleeding, 3) risk of treatment cessation, 4) low proportion of patients (<1/3) undergoing PCI and stenting prior to or together with TAVI, 5) large proportion of TAVI patients (>1/3) requiring anticoagulation due to permanent AF or AF of new occurrence, in particular those with a high CHA2DS2-VASc score. In those patients on oral anticoagulation, a careful INR monitoring, with more frequent checks, is needed. The alternative prescription of novel oral anticoagulants might be considered, though there is less experience with this drug class.
Late prosthesis embolization (LPE)
The embolization of the TAVI prosthesis from the implant site is also a very rare event. It generally occurs in the LV (89% of cases), sporadically in the ascending aorta. Risk factors for LPE are: prosthesis undersizing, or underexpansion mainly due to aortic root calcification, low implant into the LV efflux tract, bicuspid valve, large annular calcification with insufficient prosthesis anchoring, asymmetric aortic root calcification, mitral prosthetic valve, unstable prosthetic positioning, basal septal bulging. The timing varies from two days to more than one year, and symptoms are generally related to the occurrence of acute LV failure and/or cardiogenic shock. Echocardiographic findings for suspicion of LPE occurrence or short-term risk are moderate PAR in conjunction with high transvalvular gradients. The treatment of choice is emergent surgery.
Prosthesis compression
This curious complication consists of a mechanical deformation of the prosthesis due to external chest compression and has been described after attempts at cardiopulmonary resuscitation (CPR) in seven patients, only with the balloon-expandable TAVI prosthesis [6]. The incidence is probably underestimated. However, it should be looked for after CPR. A possible therapy after resuscitation, described in only one case after resuscitation, could be prosthesis re-dilation [13].
Prosthesis durability
The durability of a TAVI prosthesis should theoretically be comparable to that of a surgical bioprosthesis. In a published series, only 3.4% of patients developed a moderate stenosis at five-year FU [14]. In a different series, valve gradients remained stable over an FU of two to five years, without new AR development [15,16]. However, longer-term data are warranted.
Management problems
The large majority of TAVI patients are very old, with many comorbidities, sometimes with a low socio-cultural level, not infrequently alone or are without family support. These conditions may lead to substantial management problems which can be summarized as follows:
- Lack of family support and poor compliance to therapy and out-patient visits
Lack of help from family members in reminding the patient of the schedule of the FU visits, bringing them to the hospital, and verifying the consumption of drug. The lost appointments to FU visits may be followed by the impossibility of therapy adjustments.
- Therapy adjustments
Lack of prompt activation of diagnostic and therapeutic procedures in case of suspected complications, leading to the progressive deterioration of the clinical conditions with consequent hospitalizations.
- (Recurrent) hospitalizations
These occur frequently in general medicine divisions not specialized in the difficult management of these very complex patients, and which are wholly inadequate to handle the diagnosis and treatment of the specific TAVI complications.
As a consequence, a well-structured and complex system is needed to deal with the medical, and often the social, aspects of TAVI FU patients. This is currently not easy to organize.
Organizational problems
To the best of our knowledge no position papers or recommendations have been published to date by any scientific Society about the FU management of TAVI patients. Also, analyzing the literature, there are no important papers about this issue. There is no clear solution to some questions, and every TAVI center has its specific organization in relation to FU. In particular, some areas are not well defined:
- Who is in charge of the FU?
The Heart Team? The clinical cardiologists? The interventional cardiologists? The cardiac surgeons?
- What are the selection criteria to admit a TAVI patient into an FU program?
Age? Clinical characteristics? Late complications? All?
- What is the correct timing?
After how long from discharge? Then, every how many weeks/months? How long should a “specific” TAVI patient’s FU be?
- Which are the routine tests to be performed?
Certainly a careful clinical examination, ECG and echocardiogram, but what else? Which blood tests? And which other tests (such as Holter monitoring in LBBB), and how often?
- Which outcome criteria have to be utilized?
The VARC-2 criteria? Others?
- How should contact with GPs and referring cardiologists be organized?
- How is it possible to link TAVI patients up with social services?
Is it possible to organize a home care service for very frail patients unable to go to the hospital? What may be the interaction with social services for the problems of these subjects which are not strictly medical?
The “Mauriziano Experience”
In the Cardiology Department of the Mauriziano Hospital (III level Hub) in Turin, experience with the FU of TAVI patients began in 2008. A centralized TAVI-FU program was created and offered to all post-TAVI patients. The protocol, methods and preliminary results were presented at ESC 2015 and TCT 2015 (Aranzulla et al), and the full experience (the first, and unique to date) will be published soon.
Here we show our solutions to the aforementioned issues, some results obtained and the main criticisms we faced during our experience. In our structure, the FU of TAVI patients is organized and conducted in a specialized clinic of the Cardiovascular Division. Clinical visits are performed by the interventional cardiologists with the aid of a cardiologist experienced in TAVI imaging, mainly echo, and also involved in the procedure. During every visit clinical status is assessed, as well as compliance to therapy. The latest blood exams of the patient are evaluated and an ECG and echocardiogram are performed. Every complex patient is managed in tight collaboration with cardiac surgeons, nephrologists, immunologists, oncologists, geriatricians and any other specialists (including the psychologist) who may be needed for the solution of the specific comorbidities. The FU is organized by planning the timing of the first visit (varying from 30 days to three months according to the clinical status) at discharge.
Every patient is followed up for 12 months, then with periodic visits. After this period, the patient is addressed, according to the patient’s preference, either to our clinical cardiologists or to the referral cardiologist/cardiology center and to the GP. Those patients who do not adhere to out-patient visits are followed up by phone calls and by direct contact with the referring physicians (Figure 1). In total, we observed more than 150 patients and we experienced a high rate of adherence (>90%). One of the main criticisms consisted in the delay of the first observation of the patients, often after the first month. We did not wish to stress a very old patient with difficult transportation problems by asking them to come back to the hospital after a short period at home and after a sometimes long and complex hospitalization for the preparation for and for the recovery after the procedure. However, it was really during this period that we observed a high percentage (24%) of deaths. Had we planned an earlier patient evaluation, at least some of the complications could have been identified and probably corrected.
Figure 1. Flow chart of the Mauriziano TAVI follow-up program.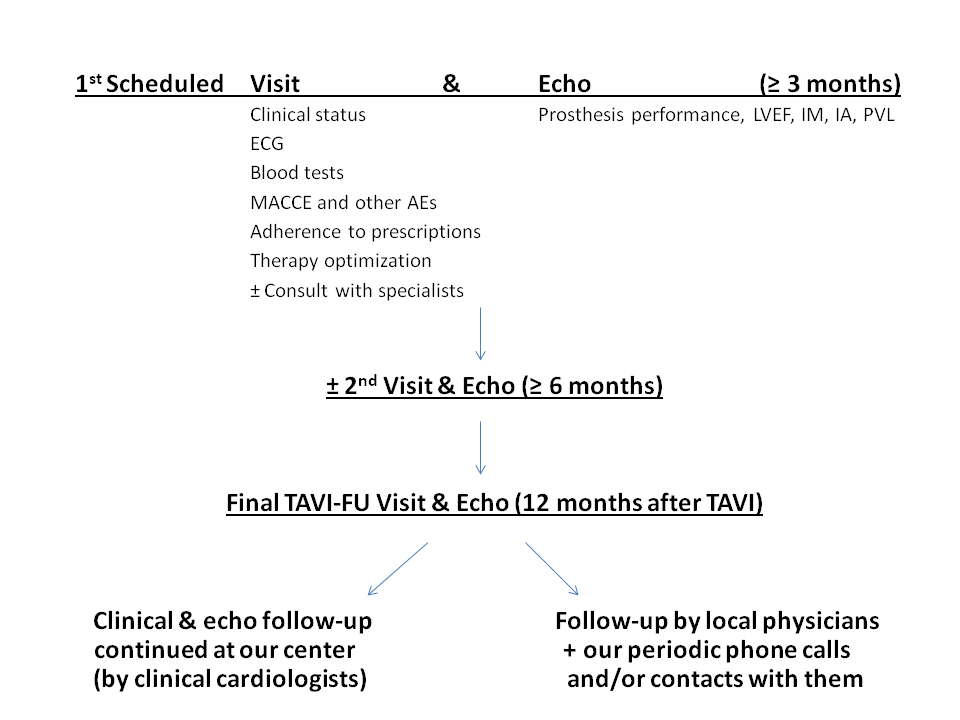
Conclusions
The care of TAVI patients does not end with the TAVI procedure. An intensive and well-organized FU should be mandatory, certainly for the early complicated patients, but also ideally for every TAVI patient. Some issues are still a matter of debate. Probably an early visit (less than one month from discharge) is an essential part of all FU procedures, as suggested by our experience. We need some clear statement such as a position paper by the international scientific societies about this very challenging topic.
This article was supported by an unrestricted educational grant from Edwards Lifesciences.





 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.