Background
Co-existence of hypertension and diabetes increases the risk of renal and other target organ damage, and heads to greater incidence of cardiac events and mortality. When renal dysfunction starts to set in, proteinuria and microalbuminuria are predictors of development of renal and cardiovascular (CV) complications, -events and mortality (1,2,3). Therefore, detecting microalbuminuria, just as scientific guidelines recommend, will screen for renal disease even in its early stages.
Strong evidence from the 1980’s on, have put forward that suppressors of the renin-angiotensin-aldosterone system (RAAS) may have specific renal protective properties. They are preferred agents both in monotherapy or as components of combination therapy (4). However, CV disease may still progress under chronic RAAS blockade (5), and therefore microalbuminuria, as predictor of both CV and renal disease, should continue to be monitored in patients who are under chronic RAAS blockade therapy.
1 - Microalbuminuria is a CV risk factor in hypertensive patients
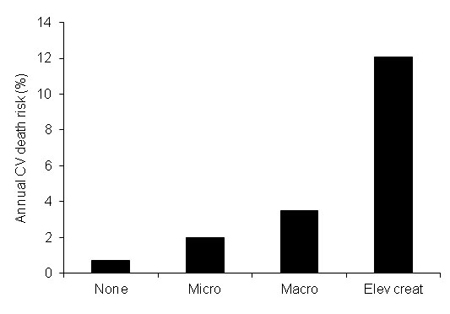
Renal disease is a complication of diabetes and hypertension - which together make up the most common causes of chronic kidney disease (CKD) (6). Furthermore, there is growing evidence revealing the continuous relationship between worsening in renal function and CV disease and a sharp increase in risk of CV morbidity and mortality (7) (Fig. 1). The actual fact of composite interactions between CV disease, chronic kidney disease (CKD) and diabetes are becoming more widely accepted, however they are not completely understood, as yet (6).
Stratification of CV risk as well, in hypertensive patients in particular, has also been considered increasingly relevant: markers suggesting preclinical renal damage are also able to detect CV disease, and moreover be used to predict CV morbidity and mortality.
Initial clinical evidence of this was provided by observational studies in high risk patients. The HOPE trial (8), confirmed the predictive value of microalbuminuria in the development of overt nephropathy and CV mortality, with a similar prognostic capability than that of a previous coronary event, both in diabetic and non-diabetic patients.
In parallel, importance of early detection of renal damage in the general population, as well as in patients with essential hypertension independently of blood pressure values, namely, by testing microalbuminuria, has been widely reported on. A great example of this was a collaborative meta-analysis of general population cohorts involving more than 1 million participants, demonstrated a direct relationship between renal dysfunction and CV risk: Estimated glomerular filtration rate <60 ml/ min/1.73 m2 and an albumin-to-creatinine ratio =1.1 mg/mmol (=10 mg/g)- the lower limit of high-normal albuminuria - were both independent predictors of mortality risk in the general population. An elevated estimated glomerular filtration rate and albumin-to-creatinine ratio each significantly increased mortality rate, without evidence of interaction between each measurement (9).
Taken together, data demonstrating the composite interactions between CV disease, chronic kidney disease and diabetes, relevance of stratification of CV risk, and the predictive value of microalbuminuria in the development of overt nephropathy and CV mortality confirm the importance of microalbuminuria as CV risk factor in hypertensive patients.
2 - RAAS blockade is the elective treatment in patients with microalbuminuria
Suppression of the RAAS is widely viewed as positive therapy to help improve renal outcome and especially helps diminish micro and macroalbuminuria. Both angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs) have renoprotective properties as has been shown in placebo-controlled, randomised trials and meta-analyses. They are able to reduce all-cause mortality, especially in patients with diabetic nephropathy (10,11,12). Thus, in patients with increased excretion of albumin in urine, RAAS blockade is essential, both to obtain controlled blood pressure (BP) and reduced albuminuria (3) which will in turn protect renal function and delay development of end-stage renal disease. That is especially the case in patients with macroalbuminuria (12,13).
In parallel, the protective function of RAAS suppression in overt CV disease was shown in patients with heart failure, post-myocardial infarction and in individuals with high global CV risk, and is especially suited to subjects with CV and renal disease.
Added to the extensively proven antihypertensive effectiveness of RAAS suppressors (3) these findings have lead to large utilisation of ACEi and ARBs, mainly in the premature stages of the CV continuum, when only a clustering of risk factors accompany BP elevation, without evidence of target organ damage or established CV disease.
3 - RAAS blockade does not rule out new-onset or progression of albuminuria
We have recently published a retrospective study on the evolution of albuminuria in a cohort of 1433 patients (mean age 60.5 years; 50.3% male), that were admitted into our Hypertension Unit (14). Patients had in common previous therapy with an ACEi or an ARB, at appropriate doses, alone or in combination with other antihypertensive drugs, for a minimum of two years prior to arrival in our Center, and they were followed for three years with maintained RAAS suppression.
a) Microalbuminuria When patients first arrived at our Unit, they presented with a high prevalence of albuminuria: 16.4% microalbuminuric and 4% macroalbuminuric. Even though patients received optimal therapy during the three years of follow-up, prevalence of albuminuria during that time increased.
b) Relation to CV disease 15.8% of patients presented previous CV disease at baseline and 4.6% developed new CV events
- Among patients with normoalbuminuria or high-normal albuminuria at baseline, progression to micro or macroalbuminuria was significantly higher in patients with previous CV disease than in those without: 17.2% versus 9.9% (p=0.003).
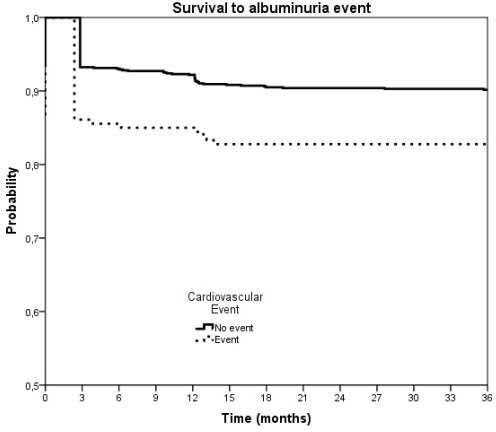
- Albuminuria progression rate was higher in patients presenting new CV events during follow-up than in those without it: 18.9% versus 10.7%, (p=0.057) (Fig. 2).
c) De novo microalbuminuria appeared in a further 16.1% of normoalbuminuric patients while an additional 1.0% developed macroalbuminuria.
- Microalbuminuria enhancement essentially took place during the first year, mainly because 15% of the normoalbuminuric patients at baseline were at the high-normal range of albuminuria (10-15 mg/day for men and 20-30 mg/day for women), near the microalbuminuria range.
- With 54-56% of patients reaching adequate BP thresholds our data confirmed that optimal long-term BP control does not exclude the risk of developing de novo microalbuminuria even in the context of supposed “satisfactory” RAAS blockade. High risk hypertensive patients with overt CV disease may develop microalbuminuria ealrier than expected.
- Factors that promoted de novo microalbuminuria were both glycemia and BP control, and were determined in our study by the number of drugs needed to obtain BP thresholds. The values of serum creatinine and baseline range of albumin were also contributing factors. Albuminuria was accompanied by a significantly higher percentage of creatinine clearance inferior to 60 ml/min/1.73m2.
4 - How to improve management of albuminuria
- Tighter BP control helps to avoid the development of microalbuminuria or allows to have it regress should it have appeared. However, further to our findings, recent studies (15,16) in which important reductions in BP levels were obtained, have not obtained significant benefits in terms of prevalence of microalbuminuria.
- Dual RAAS blockade is not recommended by guidelines, especially after the results of the ONTARGET study (17), in which the combination of telmisartan added to ramipril reduced the incidence of proteinuria, but a) produced a more prompt reduction in estimated glomerular filtration rate, b) showed no benefit in terms of CV events or mortality, as well as c) an increased incidence of renal events.
- Aliskiren, an oral direct renin inhibitor, has proved to be not only effective in a) controlling BP in obese and metabolic syndrome patients, - alone or in combination with diuretics and calcium channel blockers in different stages of arterial hypertension (18), but also in b) reducing the progression of albuminuria in diabetic individuals (AVOID)(19). Moreover, aliskiren plus either an ACEi or ARBs may possibly provide greater RAAS blockade than monotherapy with either ACEIs or ARBs, and produce additive improvement in BP and clinically relevant outcomes (20). Nevertheless, more data are still needed.
- Lastly, combination of an ACEi or an ARBs with the aldosterone receptor blocker spironolactone (21) has proved positive. Spironolactone is a good antihypertensive drug even as first step in essential hypertensive patients.
However this finding may be attributable to growing evidence that primary aldosteronism is more prevalent than considered thus far. However, it might be necessary to avoid aldosterone in patients whose estimated glomerular filtration rates are low due to the risk of hyperkalemia or worsening renal function.
Conclusion:
Long-term RAAS suppression does not appear to reliably prevent progression and evolution of albuminuria both in diabetic and non-diabetic hypertensive patients. Incomplete inhibition of the RAAS may be responsible for the residual organ damage and event rates observed hypertensives, diabetics, chronic kidney disease and heart failure patients treated with ACEi or ARBs on monotherapy. Moreover baseline renal function, glycemic control and severity of hypertension are factors independently related to increased urinary albumin excretion. Even in chronic RAAS suppression, albuminuria remains a powerful predictor of cardiovascular events. In view of improving these patients’ outcomes, further research to determine whether RAAS suppression capacity is at its maximum or if it can be further enhanced is warranted.


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.