The coronary vasculature mainly consists of smooth muscle cells, endothelial cells and fibroblasts. These cell types are derivatives of the epicardium, and therefore collectively called epicardial derived cells (EPDCs) (16). The epicardium is a mesothelial monolayer of cells, which surrounds the myocardial layer of the heart. Interestingly, the epicardium is not present when the primitive heart tube initially is formed, but is added later during cardiac development. In this respect heart development is an exception since almost all developing visceral primordia are initially covered by coelomic epithelium.
The question of the origin of the proepicardium (PE)
The process of epicardialization is known to be a crucial step in heart development and raises the question of the origin of the epicardium, which is the proepicardium (PE). The PE is a multipotent progenitor population of cells that develops at the venous pole of the heart (Fig. 1). In fact, it was shown by quail-chick chimera studies that all coronary vessels are formed by proepicardial cells (10). The distal part of the outflow tract however is covered by cells of the cephalic pericardium and not by proepicardial cells (17).
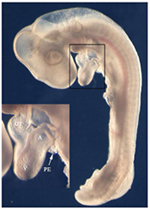
Fig. 1: Chick embryo at stage HH 17 with a looped heart displaying a cluster of proepicardial cells at the venous pole (arrow). A: atrium, OT: outflow tract, PE: proepicardium, V: ventricle.
The PE is a transient structure and displays a characteristic morphology with villous epithelial protrusions filled with mesenchymal cells . In the chick, the first PE cells become visible at HH stage 15 on the right sinus. These cells are highly proliferative and form the PE at HH stage 17, which subsequently attaches to the dorsal side of the embryonic ventricle. This epicardialization of the heart is accomplished via a secondary tissue bridge, along which the PE cells reach the surface of the heart (11). After the epicardium is formed, these cells undergo an epithelial-mesenchymal transition and invade the myocardium, where they differentiate into smooth muscle cells, endothelial cells and fibroblasts to form the coronary vessels and connective tissue, respectively (Fig.2). Furthermore, absence of the epicardium leads to retarded growth of the myocardial ventricular wall, indicating that myocardial growth relies on promoting signals from the epicardium (12).
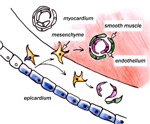
Fig.2: Epithelial-mesenchymal transition of epicardial cells and differentiation into smooth muscle and endothelial cells, which form the coronary vasculature.
Although these general developmental steps are quite similar among vertebrates there are a number of significant differences, particularly between mouse and chick, which, with regard to epicardial development, thus far are the best-studied organisms. In the chick, the PE develops on the right sinus horn, whereas on the left sinus only a small developmentally retarded PE is formed, that does not contribute to epicardium formation, but ultimately undergoes apoptosis. It was recently shown, that the PE is a target of an inductive right-sided signaling pathway, which involves FGF8/Snai1 (22). Moreover repression of PE formation via the left-sided Nodal/Pitx2 pathway is probably not important. However, in the mouse, the PE develops bilaterally and does not form a secondary tissue bridge that is connected to the heart as in the chick (23). In contrast to the secondary tissue bridge in the chick embryo, the murine PE cells reach the ventricle in clusters of epithelial vesicles, which float through the pericardial cavity and leave an epicardial “patchwork” on the heart, which finally forms the epicardium (6). Investigation of human embryos revealed similarities between the PE anlagen of mouse and man (11).
Asymmetric PE development, first observed in the chick, is also observed in lower vertebrate model organisms
Strikingly, asymmetric PE development, which was first observed in the chick is not the exception, but is also observed in lower vertebrate model organisms like Xenopus laevis. In Xenopus, the PE develops on the right side similar to the chick and the cells reach the heart over a tissue bridge (7). In the jawless lamprey, PE cells develop bilaterally, but only the right side forms a tissue bridge to colonize the ventricle (18). A similar observation was made in the dogfish (Scyliorhinus canicula), which belongs to the primitive gnathostomata (14). It remains unclear if PE cells colonize the zebrafish heart with the help of a tissue bridge, or PE cells reach the heart via a cystic cell transfer mode.
Recent data suggest the presence of right-sided asymmetric expression of PE marker genes, however bilateral expression domains are present in zebrafish mutants with a cardia bifida phenotype (24). Whether cardia bifida leads to a left-sided ventricle, which is devoid of an epicardium as in the chick (23), has not been further analyzed. Until now, the symmetric, bilateral proepicardial development in the mouse represents an exception rather than the rule among animal models of heart development. In fact, there is evidence in the chick, that epicardialization is strongly biased by a right-sided signaling pathway (22). The extent to which this concept is realized by a similar set of signaling molecules and transcripton factors in other vertebrates will be investigated in the future.
The formation of the PE and the epicardium: a dynamic and progressive process
The formation of the PE and the epicardium is a very dynamic and progressive process, which involves a number of transcription factors. Members of the T-Box family of transcription factors as well as the Wilm´s Tumor Suppressor WT1 are among the most prominent PE marker genes. Especially TBX18 and WT1 expression can be detected in PE cells of mouse, chick, frog and zebrafish (4; 7; 21; 23; 24). Although the role of these marker genes is still not fully understood, there is evidence that TBX18 and WT1 play an important role during induction of the PE (21; 22). WT1 is expressed in EPDCs that invade the myocardium and is downregulated when these cells differentiate into coronary blood vessel cells (16). Furthermore, WT1 becomes re-expressed in coronary blood vessel cells of rat hearts under hypoxic conditions (27). TBX5 was shown to play a crucial role in the migratory activity of PE cells and subsequent epicardium formation (5).
Besides these transcription factors, there are a number of additional factors displaying proepicardial expression domains. CFC is a competence factor for NODAL/ GDF signaling with strong expression in the PE and the epicardium, but its function in the PE/epicardial context remains unclear (21). Members of the BMP (Bone Morphogenetic Protein) growth factor family, including BMP2/4 are not only expressed in the sinus venosus and the PE, but are also able to modulate the expression of TBX18 in vitro and in vivo in a dose-dependent manner (21). Furthermore, BMP signaling is sufficient to induce differentiation of cardiac myocytes in proepicardial cultures (8; 21).
The cardiomyogenic potential of PE cells an indication that they were once part of the heart forming fields.
The cardiomyogenic potential of PE cells is an indication that they were once part of the heart forming fields. This idea is supported by the fact, that PE cells, together with myocardial and endocardial cells, are derived from Mesp1 expressing progenitors (20). Although the exact origin of these cells is not known, one might suggest, they are likely to reach the venous pole after heart tube formation. It is known, that the sinus venosus is a very dynamic region, whose patterning relies on GATA4, BMP4 and TBX18 (2; 19). After the PE progenitors have reached the sinus region, they are not recruited to become sinoatrial myocardium, but are destined to form the PE. At least in the chick there is evidence, that SNAI1 under the control of FGF8 might be involved in this developmental process (22).
In addition to BMPs, members of the FGF family (Fibroblast Growth Factor) are involved in PE development. FGF2 and FGF10 both display right-sided expression of proepicardial cells. Interestingly, these factors do not regulate the expression of PE marker genes like TBX18 or WT1, but are crucial for proliferation and survival of PE cells in the chick embryo. The loss of FGF signaling subsequently leads to apoptosis in the right sinus (26).
Since it was shown that proepicardial cells strongly contribute to the coronary blood vessel system (10; 13; 15), the vasculogenic potential of PE cells is a topic of intensive research. Studies in the zebrafish revealed an FGF-induced activation of the epicardium after apical myocardial injury, which leads to mobilization of epicardial cells accompanied by a significant re-expression of TBX18 and RALDH2 and ultimately leading to a neovascularization of the regenerated region (9).
Thymosinß4 is responsible for restructuring the cytoskeleton and migration of cells in the mouse
In the mouse, a protein called Thymosinß4 is responsible for restructuring the cytoskeleton and migration of cells. This factor has the capacity to stimulate the growth of epicardial explants and also to induce vasculogenesis in murine ischemic hearts (25). Furthermore, recent Cre lox studies claim a myocardial contribution from the pool of proepicardial cells, which express TBX18 and WT1 (1; 28). These findings caused an ongoing debate, since TBX18 is also expressed in the myocardium (3). However, the concept that epicardial cells contribute to the myocardium does not fit into the context of previous studies, which were not able to detect such contribution (10). Therefore it will be necessary to reinvestigate this phenomenon to solve this discrepancy.
Conclusion:
Taking all this into consideration, the insight into the developmental processes, which underlie the formation of coronary blood vessels, will be required to develop novel therapeutic strategies particularly for the ischemic heart.

 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.