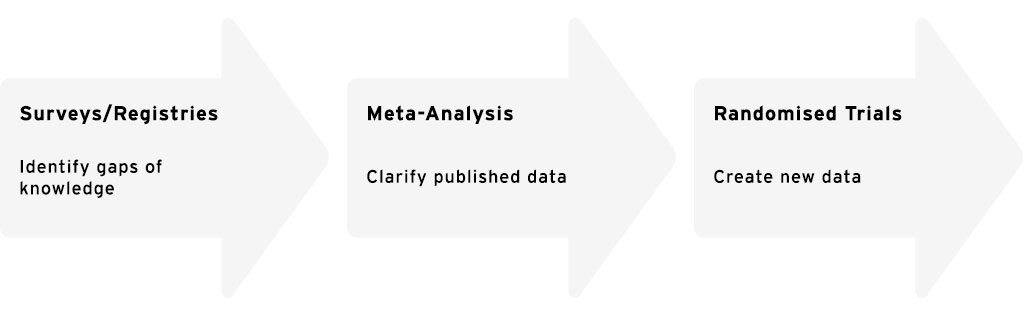
The SIC conducts several types of surveys including centre-based (addressing the EHRA Scientific Research Network), physician-based and patient-based, together with new initiatives, such as systematic reviews and meta-analysis.
The EHRA 2022-2024 Scientific Initiatives Committee Members are:
Prof. Julian Chun (Chair), Dr. Sergio Castrejon (Co-Chair), Dr. Laura Perrotta, Prof. Andreas Metzner, Prof. Laurent Roten, Assoc. Prof. Piotr Futyma, Assoc. Prof. Arian Sultan, Prof. Sergio Richter, Dr. Giacomo Mugnai, Dr. Ante Anic, Prof. Federico Migliore, Prof. Giulio Conte, Dr. Antonio Berruezo Sanchez, and Dr. Rui Providencia
2022-2024 Strategy
The EHRA Scientific Initiative Committee (SIC) calls for your ideas and your scientific international cooperation.
The committee invites you to propose your ideas for surveys and meta-analyses to help close the gap(s) of evidence in the field of electrophysiology or pacing, with emphasis on:
- Arrhythmia management/patient care
- Implementation of technological innovations
- Arrhythmias and heart failure
If you would like to submit a proposal which could fit into the above mentioned areas, the SIC committee would be delighted to consider and review it.
In addition, the methodology of this clinical research will now call upon three stages: inventory, identification and correction of potential deficiencies, mid-term re-evaluation.

 The results of these surveys enable us to gather some valuable and essential information, thanks to participating centres who regularly complete the surveys and entrust us with their data.
The results of these surveys enable us to gather some valuable and essential information, thanks to participating centres who regularly complete the surveys and entrust us with their data. Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.