Abbreviations
AI aortoiliac
AAA abdominal aortic aneurysms
ABI ankle-brachial index
CERAB covered endovascular reconstruction of aortic bifurcation
CF colour-flow
CFA common femoral artery
CLI critical limb ischaemia
CLTI critical limb-threatening ischaemia
CTA computed tomography angiography
DCB drug-coated balloons
DES drug-eluting stents
DP dorsalis pedis artery
DSA digital subtraction angiography
ER endovascular repair
GLASS Global Limb Anatomic Staging System
GSV great saphenous vein
HyR hybrid repair
IC intermittent claudication
IM inframalleolar arteries
ITF intermediate technical failure
LBP limb-based patency
MACE major adverse cardiovascular events
MRA magnetic resonance angiography
OR open repair
PAD peripheral arterial disease
PBA plain balloon angioplasty
PFA profunda femoris artery
TAP target arterial path
Introduction
Atherosclerosis is the main cause of peripheral arterial disease (PAD) involving the lower limbs with higher prevalence in men than in women, increasing with age from 0.9% in subjects aged <50 years to 14.5% in individuals >70 years. It may coexist with multiple vascular involvement from coronary to cerebrovascular, visceral and renal arteries representing a significant disease burden for health care [1]. Two hundred million people worldwide suffer from PAD and its prevalence increased by 23.5% in the first decade of the 21st century. The annual incidence of major amputations, particularly in diabetics, is a major public health problem associated with very poor outcomes: two years after below-knee amputation 30% of patients died, 15% had an above-knee amputation and/or required a contralateral amputation, and only 40% achieved full mobility [2].
Chronic distal embolisation from aortic and popliteal aneurysms may result in severe ischaemic syndromes requiring revascularisation, as in atherosclerotic disease. Other causes of PAD in patients aged <50 years such as Buerger’s disease, non-specific aorto-arteritis, Takayasu’s disease, arterial entrapment syndromes, fibrodysplasia and other uncommon arteriopathies will not be discussed in this paper.
Atherosclerosis progression to severe ulcerated and calcified lesions with flow-restricting stenosis and occlusions is associated with major adverse cardiovascular events (MACE). Modern medical treatment aims to modulate its progression, to reduce MACE and improve patient survival. Control of common risk factors associated with PAD such as hypertension, diabetes mellitus, chronic kidney disease, dyslipidaemia, together with smoking cessation, promotion of heathy lifestyle plus antiplatelet medication and appropriate revascularisation are the cornerstones of modern management.
Atherosclerotic PAD may be segmental or diffuse from the infrarenal aorta and iliac arteries to the femoral, popliteal and tibial arteries and extending to the foot arteries.
Around 75% of patients are asymptomatic, only recognised by the absence of peripheral pulses on complete clinical examination or reduced ankle-brachial pressure index (ABI) at rest or following a standard exercise test; 10% may present with intermittent claudication (IC) and 15% with more advanced ischaemic changes from nocturnal rest pain to ulceration and gangrene on the lower limbs [3]. Amputation risk ranges from 1% to 3% in five years in claudicants but may reach 30-40% in more advanced stages of limb ischaemia without successful revascularisation.
Grading of signs and symptoms according to their clinical severity provided a standardised approach for diagnosis and treatment guidance [4,5].
PAD is, therefore, a marker of high-risk cardiovascular morbidity and mortality and of increased risk of limb loss. Successful management requires a patient-tailored approach based on individual clinical risk, full mapping of the arterial disease and analysis of the expected risk-benefit ratio from therapeutical interventions.
Patient assessment
Three main steps are required:
- Staging of limb ischaemic severity
- Evaluation of patient risk
- Knowledge of the anatomy of occlusive disease and hemodynamic impairment.
Staging of limb ischaemic severity
Intermittent claudication (IC), defined as muscle pain usually starting in the calves, increasing with walking effort and disappearing by rest, should be distinguished from arthritis, lumbar spinal stenosis and from severe deep venous insufficiency where pain relief requires limb elevation and is associated with leg and foot oedema. With proximal arterial obstruction (i.e., the aortoiliac [AI] segment), proximal extension of calf muscle pain into the thighs and buttocks may occur; isolated buttock claudication is rare and associated with bilateral hypogastric severe occlusive disease. Plantar claudication is present with isolated leg arterial involvement. It is more common in diabetics and younger patients with non-atherosclerotic occlusive disease.
IC is usually considered tolerated or non-limiting if the patient can still manage his/her daily activities (stage IIA) and incapacitating or limiting if performance of the usual activities is impaired (stage IIB) [4]. Absence of peripheral pulses plus an ABI <0.9 in resting conditions or a post-exercise reduction of ankle pressure >30 mmHg or of resting ABI >20% are diagnostic of PAD. In patients with diabetes and end-stage renal disease the presence of highly calcified arteries is common and measurement of toe pressure with ultrasound or plethysmography is useful.
Rest pain defined as nocturnal pain localised in the foot relieved by foot dependency or standing (Fontaine stage III and Rutherford stage II) should be distinguished from muscle cramping, arthritis and/or foot infection and is a marker of increased severity of limb ischaemia.
Critical limb ischaemia (CLI) identifies patients with nocturnal rest pain associated with reduced ankle pressure <50 mmHg and/or toe pressure <30 mmHg and ischaemic lesions such as ulcerations or gangrene at an impending risk of limb loss without an adequate revascularisation procedure [6].
Critical limb-threatening ischaemia (CLTI) was recently introduced to encompass ulceration, infection, neuropathy and lesions with delayed healing processes (Table 1) with increased risk of major limb loss without successful revascularisation [6,7].
Table 1. Critical limb-threatening ischaemia (CLTI).
|
Critical limb-threatening ischaemia |
|---|
| Ischaemic rest pain >2 weeks plus |
| Ankle pressure <50 mmHg |
| Toe pressure <30 mmHg |
| ABI <0.4 |
| Diabetic foot / lower limb ulceration for 2 weeks |
| Gangrene involving any portion of the lower limb or foot |
ABI: ankle-brachial index
Use of the WIFI (Wound, Ischaemia, and Foot Infection) classification [7] combining wound assessment (W), severity of ischaemia by ankle and/or toe pressures (I), and type and extension of foot infection (FI) provides more accurate prediction of limb loss, improving patient selection for a revascularisation procedure.
Patient risk estimation
Full clinical and laboratory general evaluation should be carried in all patients with PAD to identify uncontrolled risk factors, major organ involvement and underlying concomitant disease. A 12-lead ECG and echocardiogram are recommended to diagnose severe and unstable heart disease requiring urgent treatment. Dobutamine stress echocardiography and/or isotopic scintigraphy are important to identify silent coronary disease and for selection for coronary angiography and revascularisation prior to PAD invasive treatment.
Concomitant multivessel disease involving extracranial carotids, visceral and renal arteries is not uncommon and selective treatment may be indicated. Symptomatic unstable carotid stenosis >70% should be dealt with previously or simultaneously [8]. However, for asymptomatic carotid disease, the treatment decision should be individualised according to its haemodynamic severity, presence of biomarkers of active carotid plaque, presence of previous silent infarcts, embolisation on transcranial Doppler and local surgical results [8,9]. Concomitant abdominal aortic aneurysms (AAA) >5.5 cm and popliteal aneurysms are not uncommon, requiring treatment to prevent rupture and/or distal embolisation.
Anatomy of arterial disease
Mapping of arterial disease from infrarenal aorta to foot arteries must be stratified according to the arterial segments involved:
- proximal corresponding to aorta and iliac arteries
- femoral to common femoral artery and its bifurcation
- infrainguinal to superficial femoral and popliteal arteries
- infragenicular for popliteal bifurcation and tibial and peroneal arteries
- inframalleolar (IM) including foot arteries and status of the plantar arch especially relevant in CTLI and diabetic patients
Colour-flow (CF) duplex scanning combining morphology and functional assessment of stenosis/occlusions and diagnosis of concomitant arterial aneurysms should be the first-line method for full non-invasive evaluation of the arterial system. CF duplex scanning is important for surveillance programmes following endovascular or open revascularisation to detect early correctable complications and to improve its durability.
More invasive arterial imaging should be reserved when interventional endovascular or open repair is considered.
Computed tomography angiography (CTA) is an objective non-operator-dependent arterial imaging with high sensitivity and specificity for AI stenoses >50%, for the femoropopliteal region and below-the-knee arteries. CTA provides visualisation of the type and extension of calcifications and the status of previous open or endovascular revascularisations. Allergic reactions and contrast-related nephropathy are limitations to its widespread use, but N-acetylcysteine has been shown to reduce contrast-mediated kidney injury.
Magnetic resonance angiography (MRA) with gadolinium enhancement also has an excellent sensitivity and specificity compared to CTA and conventional angiography. Its limitations are the presence of pacemakers or metal implants (including some early stents), patients with claustrophobia and severe renal failure (glomerular filtration rate [GFR], 30 mL/min per 1.73 m²). Slow flow states with significant venous overlap obscuring arterial anatomy and unclear visualisation of arterial calcifications are limitations for planning endovascular or open surgical repair.
CTA and MRA provide satisfactory imaging in the majority of cases, with less radiation and avoiding complications with diagnostic arteriography reported in 1% of cases; however, visualisation of crural and plantar arteries in severe multi-segmental disease with reduced distal arterial flow is an important limitation requiring angiography [10,11].
Digital subtraction angiography (DSA) from aorta to foot arteries through retrograde transfemoral catheterisation continues to be the gold standard imaging technology for PAD assessment. Crossover techniques allow direct antegrade flow imaging from one side to the other. If the femoral access is not possible, transradial and transbrachial/axillary approaches or direct antegrade catheterisation may be used.
In modern practice, DSA is now reserved for patients undergoing interventions as the first step of an endovascular procedure.
Management
Conservative treatment should be the first-line treatment in PAD patients; its discussion is beyond the scope of this paper.
For claudication, active smoking cessation, risk factor control, standardised exercise programmes and antiplatelet medication should be implemented for all claudicants to provide stabilisation and/or improvement in walking distance.
Revascularisation should be reserved for patients with
- severe limiting claudication with reduced quality of life;
- progressive clinical deterioration irrespective of adequate medical treatment;
- presence of rest pain and/or CLTI.
Revascularisation aims to restore direct pulsatile flow to the distal extremity either through endovascular repair (ER) with intraluminal recanalisation, angioplasty with or without stenting, or by open repair (OR) with endarterectomy for localised stenosis/occlusions or bypass grafting for more extensive lesions. Hybrid repair (HyR) combining both methods is a useful alternative with excellent outcomes.
Both ER and OR expertise should be available in all centres offering invasive treatment for PAD patients. Multidisciplinary cooperation for management of CLTI patients and an aggressive policy for revascularisation are essential to improve patient survival and to reduce the number of major amputations.
New developments in endovascular technologies have challenged established concepts limiting endovascular options to A and B lesions [2] and expanded the role of ER for more complex and longer lesions with results comparable to OR [12].
For patients with CLTI, a new Global Limb Anatomic Staging System (GLASS) [7] was introduced to improve selection between ER and OR. It is a complex system based upon several factors:
- identification of the target arterial path (TAP), defined as the best direct flow route to the leg and foot arteries
- segmental grading from AI to common femoral bifurcation and infrainguinal disease
- presence and type of calcification
- status of the IM arteries
- estimated limb-based patency (LBP)
- disease complexity from I to III grades based upon estimations of intermediate technical failure (ITF) and LBP, relevant for femoropopliteal and crural disease
Selection for endovascular or open surgery depends on location, morphology and extension of occlusive arterial disease.
Aortoiliac occlusive disease
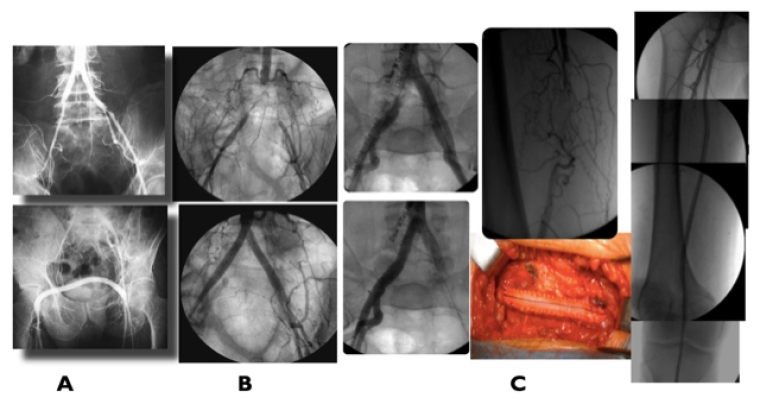
Guidance for revascularisation based on morphology and extension of disease where ER was the first option only for A and B lesions and OR/HyR for C and D lesions (Figure 1A) has radically changed. New and better guidewires for recanalisation of chronic occlusions, primary stenting and the use of covered stents for distal aorta and bilateral iliac occlusions have expanded endovascular options to complex lesions [12,13,14], including re-fashioning of new AI bifurcation – CERAB for covered endovascular reconstruction of aortic bifurcation - and complete AI occlusions (Figure 1B) with excellent geometrical reconstruction, prevention of thrombus fragmentation and migration through the bare stent cells and midterm and long-term outcomes comparable to OR. HyR with local endarterectomy with profundaplasty is useful to improve outflow especially in multi-segmental occlusive disease and/or severe stenosis of the profunda femoris and to provide an access (Figure 1C) for endovascular treatment of multi-segmental arterial disease.
Figure 1. Proximal revascularisation
A) Hybrid repair: iliac stenting + femoro-femoral crossover bypass for left external iliac stenosis plus right superficial femoral occlusion. B) CERAB for AI complete occlusion. C) Multilevel right common iliac treated by hybrid procedure: right common femoral artery endarterectomy and profundaplasty + iliac stenting and superficial femoral artery stenting for long occlusion.
OR can be anatomic (direct) - local endarterectomy or bypass between anatomical contiguous arteries - or extra-anatomic (indirect) with the use of a bypass between two non-contiguous arteries.
Aorto-femoral bifurcated bypass in patients fit for surgery was considered the gold standard with excellent long-term assisted primary and secondary patency rates, although with early morbidity and a hospital mortality of 2.4%, sexual dysfunction, increased risk of bowel obstruction due to adhesion formation, incisional hernias and late graft-related complications such as anastomotic aneurysms (5-10%), aorto-enteric fistulae (1-2%) and late infection [15]. For high-risk patients, unfit for surgery and not suitable for ER, extra-anatomic revascularisations continue to be a viable option because of lower morbidity and mortality and acceptable late outcomes [16].
ER became the first option for AI revascularisation (including extensive occlusions) but OR should not be a lost art. Its main indications are: i) small aortic syndrome; ii) reduced calibre iliac arteries more common in women; iii) extensive disease involving the common femoral artery; iv) diffuse calcification; v) visceral occlusive disease not amenable to ER; and vi) failure of ER, especially if redo ER may result in compromise of major femoral outflow rendering a potential limb-saving open procedure difficult or impossible.
Common femoral occlusive disease
The common femoral artery (CFA) and the profunda femoris artery (PFA) with its network provide a bridge for collateral flow in diseased superficial femoral arteries which is essential for a successful proximal revascularisation in multi-segmental disease. Occlusive disease involving the CFA is not uncommon with bulky calcified and ulcerated plaques – coral-reef lesions – frequently involving the ostium and initial segment of the profunda. OR with local endarterectomy and patch angioplasty enlarging the ostium and the proximal segment of the profunda has clear haemodynamic benefit and is associated with low mortality and morbidity and excellent long-term durability [17,18].
Profundaplasty or prosthetic graft interposition to a more distal disease-free profunda segment continues to be the preferred method to deal with severe CFA disease as an isolated procedure or as a hybrid procedure with proximal and/or distal ER for combined disease. CFA and PFA preservation is an absolute requirement in advanced limb ischaemia with a long-term beneficial impact on limb preservation after failure of distal revascularisation or to achieve a successful below-the-knee amputation stump.
Endovascular interventions may provide a safe alternative in selected patients with high surgical risk, hostile groin due to infection or after endarterectomy, but its long-term durability is still controversial [18].
Femoropopliteal occlusive disease
Revascularisation in femoropopliteal disease is not as durable as in the AI segment. Superficial femoral artery (SFA) stenosis or occlusion is the most common lesion in PAD and without associated disease is well tolerated and does not require intervention. Direct inflow through the CFA and profunda femoris, patent distal popliteal and at least one patent leg artery providing a direct flow pathway to the foot and patent plantar arch are key factors for success and durability of revascularisation, especially in CLTI patients [7]. The use of drug-eluting stents (DES) to reduce restenosis and the need for repeated target vessel reintervention improved the long-term durability of ER when compared with plain balloon angioplasty (PBA) [19].
For claudication, open or endovascular revascularisation in isolated femoropopliteal disease is seldom advised except in severe limiting claudication with haemodynamically significant femoropopliteal disease. ER is recommended for occlusions <25 cm considering patient life expectancy, estimated risk of restenosis and need for repeated reinterventions. Retrograde recanalization through an echo-guided percutaneous approach of the distal popliteal artery is useful for recanalisation in chronic occlusions followed by angioplasty and stenting. Drug-eluting stents were associated with a significant improvement in primary patency rates, comparing favourably with 5-year patency of 67% with above-the-knee prosthetic and great saphenous vein (GSV) conduits [20].
OR should be considered the first option for longer occlusions >25 cm, the presence of multiple and diffuse lesions, with poor-quality distal popliteal/crural run-off and extensive calcifications.
For severe ischaemia (CLTI), early endovascular or open revascularisation is required to obtain pain relief, healing of ischaemic lesions and to prevent major amputation. In the BASIL trial comparing endovascular angioplasty with bypass, 70% survival at 2 years was recognised, confirming the importance of the durability of the revascularisation. Higher reintervention rates following endovascular treatment were recorded, but several drawbacks from that trial must be considered, from the lack of a clear definition of CLTI selection criteria to the preferential use of primary balloon angioplasty without stenting as the main endovascular technique [21].
Multi-segmental arterial disease with involvement of both femoropopliteal and AI segments or the distal popliteal and leg arteries is common in CLTI. Staged AI repair can be performed for limited non-progressing necrosis, provided a re-entry into the distal popliteal artery through the profunda femoris collateral system is present. With more advanced necrosis and severe haemodynamic impairment - WIFI >3 - proximal and infrainguinal revascularisation may be required to achieve successful healing of the ischaemic lesions.
A roadmap for clinical decision and strategy to choose between open or endovascular revascularisation (Table 2) should be based upon the severity of ischaemia and infection, anatomic pattern (based on TASC II classification) and presence of extensive calcification and autologous vein availability [22]. Hybrid solutions often offer valid options combining endovascular and open procedures.
For patients lacking an adequate GSV, where prosthetic or venous allografts are the only options for open revascularisation and given the inferior performance of these conduits in the below-the-knee position, endovascular intervention should be preferred if possible [22].
Table 2. Revascularisation strategy.
| Revascularisation strategy | ||
|---|---|---|
| Severity of ischaemia | major tissue loss | minor ulcer/infection |
| Anatomic pattern | long lesion/diffuse calcification | isolated/complex lesions* |
| Vein availability | GSV or good alternative vein | inadequate vein |
| SURGERY | ENDOVASCULAR | |
| HYBRID | HYBRID | |
* Provided EV is feasible
New endovascular adjuvant procedures for distal vessel preparation such as atherectomy and calcium removal devices may contribute to improve feasibility and midterm durability of ER, enlarging its scope for less favourable anatomies, particularly in the absence of a suitable venous conduit.
Use of the GLASS staging protocol to promote a more objective and standardised choice between ER and OR correlated with improved clinical outcomes for CLTI patients [7].
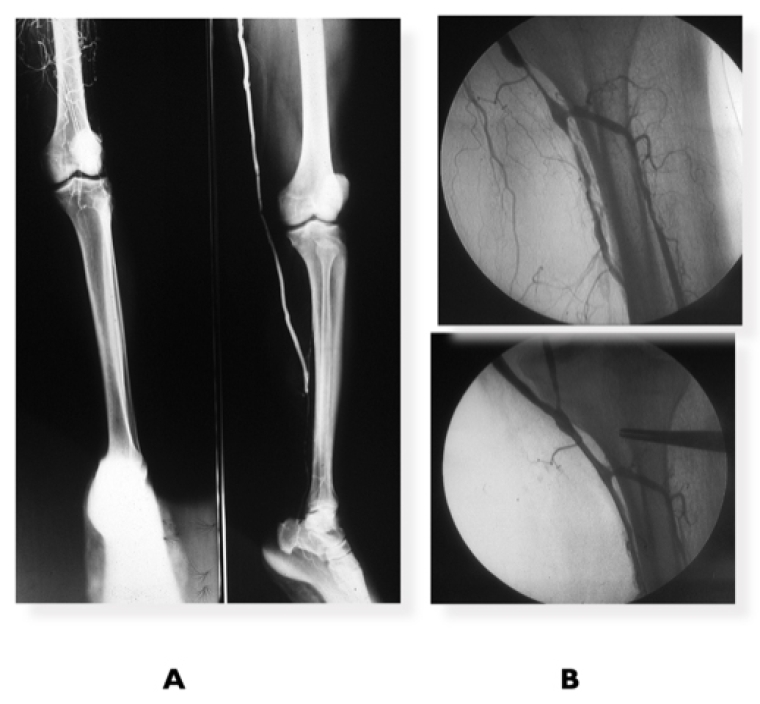
Identification of the TAP, defined as the best direct flow route to the leg and foot arteries, is paramount. It may require a hybrid procedure combining a bypass with distal infrapopliteal ER or extending the vein bypass directly to a patent crural artery connected with the foot arterial vasculature (Figure 2, A and B).
Figure 2. Femoropopliteal revascularisation
A) Diabetic patient with CLTI with extensive femoropopliteal complete occlusion + leg disease, run-off 1 vessel. Successful femoro-posterior tibial vein bypass. B) Femoropopliteal vein bypass plus popliteal/tibio-peroneal trunk PBA for CLTI.
Surveillance programmes using CF duplex scanning for ER and OR are mandatory to detect early restenosis in order to promote its prompt treatment and to prevent failure of the revascularisation procedure.
Infrapopliteal and inframalleolar occlusive disease
Isolated tibial occlusions are a very rare cause of claudication; when present it is always associated with proximal occlusive disease and its invasive treatment could be required (see above). Infrapopliteal treatment for claudication is currently not recommended [9,10].
Involvement of the tibial and peroneal arteries with diffuse calcification associated with multiple sequential stenosis and/or segmental occlusions is common in diabetics and end-stage kidney disease patients.
ER with PBA was initially recommended only for short segmental lesions, but new improved wires, low-profile catheters and retrograde fluoroscopic/echo-guided access from a patent distal artery facilitate crossing of difficult chronic total occlusions with re-entry into the true lumen and have expanded its use as a procedure of choice. Drug-coated balloons (DCB) are effective and improve angioplasty durability; however, recent trials showed no significant benefit when compared to PBA and primary DES are usually reserved as bail-out [23].
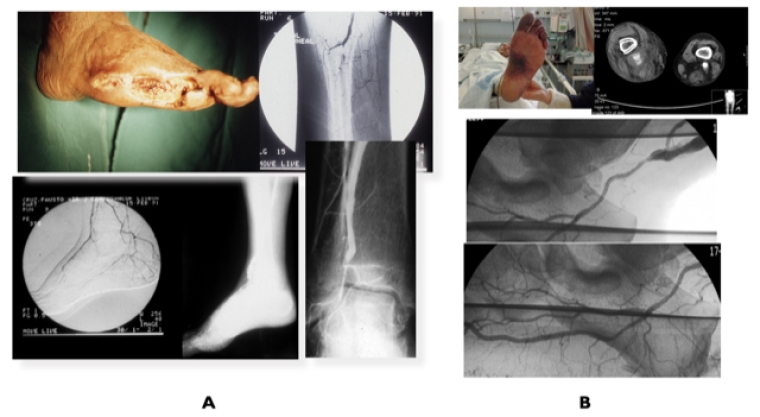
Open bypass surgery for extensive and calcified occlusions in patients with average surgical risk and a suitable autologous vein (Table 2) continues to be the preferred choice for revascularisation with an inflow source from a distal popliteal artery. In diabetic patients, a patent segment of a pedal or plantar artery can be targeted for a bypass (Figure 3, A and B). Prosthetic grafts with adjuvant techniques such as arteriovenous fistula or venous cuffs at the distal anastomosis have been used, but with poor long-term durability.
Figure 3. Revascularisation for infra-popliteal disease
A) Diabetic patient with CLTI toe amputations due to extensive long occlusions in all infrapopliteal arteries and patent dorsalis pedis (DP) artery treated by distal popliteal–DP vein bypass. B) Patient with CLTI due to distal infrapopliteal occlusions associated with chronic embolisation from a popliteal aneurysm successfully treated by SFA–plantar artery vein bypass.
As mentioned, a roadmap (Table 2) helps to guide clinical decision making for each individual patient.
Controversy concerning the use of paclitaxel-eluting devices in both infrainguinal arterial lesions was stimulated by detection of a mortality sign in a systematic review and meta-analysis of published data [24], not confirmed in subsequent publications [25]. Continuous scrutiny is advisable.
Angiosome-guided revascularisation for complex ischaemic lesions involving the proximal and mid foot aiming to maximise direct perfusion has been suggested but conflicting results comparing direct versus indirect revascularisation and excellent results from tibial, peroneal bypasses and ultra-distal revascularisation to plantar arteries or the DP artery reduced the impetus for angiosome-guided revascularisation [6,7].
Multivessel (tibial and peroneal) endovascular revascularisation is advised as first-line procedure for selected patients with advanced WIFI stages 3 and 4.
IM disease with an incomplete or absent plantar arch and an occluded DP and plantar artery – desert foot - may compromise the success of open or endovascular revascularisation and is usually designated for no-option CLTI. Endovascular interventions on the pedal arch – pedal loop technique – with recanalisation of the occluded plantar arch represent a promising technology with good early results. However, midterm durability and the need for redo procedures have yet to be determined [7].
Venous arterialisation by open surgery or through endovascular fashioned arteriovenous communication showed promising early results for limb salvage but requires confirmation in longer follow-up [7].
Adjuvant treatments such as hyperbaric O2 and vacuum therapy, prostanoids and angiogenesis play an important role to potentiate the beneficial effect of revascularisation and to avoid major limb amputation.
Conclusions
- Contemporary management of PAD patients should be centralised in dedicated centres providing multi-disciplinary cooperation for patient assessment and offering expertise in both endovascular and open repair.
- Conservative management with risk factor control, active smoking cessation, standardised exercise programme for claudicants, use of statins and antiplatelet medication should be the first-line treatment in PAD management.
- Revascularisation should be reserved only for patients with very severe limiting claudication, persisting rest pain and critical limb-threatening ischaemia.
- Preoperative mapping of the anatomy of occlusive disease from infrarenal aorta to the foot circulation is mandatory in patients requiring intervention.
- Contemporary endovascular procedures have become the first option for revascularisation even in more complex longer lesions with durability comparable to open repair.
- Severe and extensive calcification, long occlusions and poor distal run-off are persisting limitations for endovascular treatment and may also compromise a successful open repair.
- Increased mortality detected with paclitaxel-eluting devices is not directly cause-effect or dose-related; further scrutiny is advisable.
- Open surgical repair is not a lost art and it must be taught and practised as first option in low-/average-risk patients, for extensive and complex lesions with diffuse arterial calcification.
- New endovascular procedures with distal leg/foot venous arterialisation may enhance revascularisation possibilities in patients at risk of major amputation without option for conventional open or endovascular revascularisation.







 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.