Introduction
Aortic valve stenosis (AS) mainly affects elderly patients and has a poor prognosis after symptoms of dyspnoea, angina or syncope occur. Surgical aortic valve replacement (sAVR) has been the gold standard treatment for a long time but, with an ageing and an increasingly multimorbid population, the need for less invasive therapies was identified in the first European Heart Survey, where a significant number of patients was not referred or accepted for surgery.
While surgical treatment options were modified using limited access surgery and newly designed valve prostheses to improve outcomes, transcatheter aortic valve implantation (TAVI) was introduced in 2004 to further reduce surgical trauma and to avoid cardiac arrest and cardiopulmonary bypass. With increasing clinical experience and by using modern transcatheter heart valves (THVs), outcomes of TAVI have continuously improved. Early randomised controlled trials demonstrated that TAVI is a therapeutic option in patients who are inoperable [1]. Later, we also learnt from randomised studies that TAVI is an effective treatment in patients at higher risk for sAVR [2].
In the recent European guidelines on valvular heart disease, the achievements around TAVI are recognised and new recommendations for the assessment and treatment of patients with AS are provided [3]. Given the comprehensive therapeutic options available for AS, and also the variety of patients’ general and technical/anatomical risk profiles, the “Heart Team”, a multidisciplinary team of cardiologists, cardiac surgeons, anaesthetists, care of the elderly specialists and non-medical cardiac care specialists, has been placed at the centre for the decision process on the choice of the surgical/interventional treatment used in these patients. These Heart Teams are seen to operate best in Heart Valve Centres, in which comprehensive diagnostic and therapeutic options are provided to the highest standards. The treatment provided in these centres of excellence can be adjusted to individual patient needs, which should consequently improve results.
Surgical state of the art
Surgical AVR had been the only effective therapy for patients with AS until 2004, when TAVI was introduced. The aim of surgery is to immediately restore normal aortic valve function, usually with low incidence of paravalvular leakage (PVL), patient-prosthesis mismatch (PPM), atrioventricular blockage (AV block) and perioperative mortality [4,5]. While outcomes have steadily improved since sAVR was started, recently the surgical trauma could be further reduced when limited access sAVR through upper sternotomy or right thoracotomy became routine [6,7]. Even cardiopulmonary bypass times and cardiac ischaemic times, which, if elevated increase perioperative morbidity, could be further reduced using sutureless aortic valve prostheses [7].
While it is important to keep in mind that currently sAVR is the only technique to implant mechanical prostheses in younger patients (according to European guidelines to be considered in patients <60 years of age), in elderly patients biological valve prostheses have long been seen as the ideal substitute, as anticoagulation can be avoided and long-term durability is less of a concern. However, it is important to note that, using modern types of bioprostheses, valve durability can be further improved. Even in patients <60 years of age, freedom from structural valve degeneration of 50% at 17 years after sAVR using stented bioprostheses [8,9] and 70% at 15 years after aortic root replacement using stentless bioprostheses [10] has been reported. This long-term durability is convincing and paramount to achieving quality of life improvement and cost-effectiveness of aortic valve treatment in the long term. However, with an increasingly demanding patient population in terms of quality of life, including non-compliance with life-changing medical treatment such as life-long anticoagulation, the need for further development of longer-lasting bioprostheses is becoming apparent so that they can benefit younger patients. The clinical implementation of bioprostheses with a completely new design always poses a risk of early structural valve degeneration, as previously seen in surgical prostheses. However, minor adjustments, such as the modification of biological tissue treatment, using bioprostheses with well-proven stent designs, is a safer strategy in the long term [11].
Tavi state of the art
Since Alain Cribier performed the first TAVI in an inoperable patient in 2004, transcatheter valve intervention has become an established therapy for patients with AS.
Prospective randomised data from the PARTNER B cohort has demonstrated that TAVI is superior to medical therapy in inoperable patients up to five years after valve implantation [12]. A number of randomised trials have compared the outcome of TAVI versus sAVR in patients at high risk for sAVR (mean STS score 7-11%, mean logistic EuroSCORE 18-29%) [2,13]. Results up to five years have shown that TAVI is non-inferior to sAVR and those patients who are suitable candidates for transfemoral access have an additional benefit from TAVI. New data from randomised intermediate-risk patients (mean STS score 4-8%) have again demonstrated no difference in one-year mortality between TAVI and sAVR, with the lowest mortality observed in transfemoral TAVI patients [14]. However, it has also been observed that, using TAVI, major bleeding complications were reduced and PPM more easily avoided, while higher risks of vascular access complications, PVL and AV block were observed compared to sAVR.
Apart from these impressive outcomes in patients with native aortic valve disease, TAVI was also identified early on as an elegant treatment option in patients with bioprosthetic valve failure, who face repeat sAVR with its potentially increased surgical trauma [15]. While so-called valve-in-valve TAVI has not been compared to conventional sAVR in randomised studies, it has been shown that it results in excellent outcomes in higher-risk elderly patients. Haemodynamic improvements are easily achieved in larger failing bioprostheses. However, in small prostheses, TAVI, in contrast to sAVR, results in higher transprosthetic gradients and can cause PPM [16].
While these results are convincing, one needs to bear in mind that the mean age in these trials is around 80 years and that there is not much evidence available for outcomes of TAVI in younger patients. Patients included in these studies were also highly selected. Common exclusion criteria included concomitant cardiac morbidities, such as severe mitral and tricuspid valve disease and severe coronary artery disease, and anatomical relative contraindications, such as bicuspid aortic valves, inadequate aortic annulus diameter, increased risk of coronary obstruction, hypertrophic cardiomyopathy with left ventricular outflow tract obstruction and severe pulmonary hypertension. In this respect, it will also be of importance for the future to see how the availability of a greater variety of THVs, with additional features such as retrievability, smaller device diameters or the ability of anatomical deployment, will widen the technical indications for TAVI.
Regarding the durability of THVs, it is currently difficult to predict what the long-term outcome will be. While no major issues of structural valve degeneration have been reported as yet, it is important to keep in mind that elderly patients, such as those currently treated in TAVI trials, carry the lowest risk of structural valve degeneration after biological sAVR. In these patients, degeneration of conventional bioprostheses would not be expected before ten years after implantation, a time interval, which TAVI patients have not yet reached. Taking the experience from surgical bioprostheses into account, where only certain stent designs and biological materials proved to improve long-term prosthetic durability, it is of course of concern that there is such a huge variety found in THVs in terms of design, stent and biological material used. Therefore, almost certainly, and as was similarly observed in the past in conventional bioprostheses, some of the THVs currently used will almost certainly be affected by early structural degeneration at around 10 years after implantation. As a consequence, the implantation of THVs in patients aged <75 years should currently only be performed under certain circumstances, such as in patients with comorbidities which severely increase their risk for sAVR or in those included in controlled trials, where outcomes are monitored.
The concept of the heart team
The recently published European guidelines on the treatment of valvular heart disease have placed the “Heart Team”, a multidisciplinary team of cardiologists, cardiac surgeons, anaesthetists, care of the elderly physicians and non-medical cardiac care specialists, at the centre of the decision process to select the most appropriate therapy for individual patients [3]. While it is recognised that low-risk patients with AS (STS score <4%, logistic EuroSCORE I <10%) should be directly considered for sAVR and those who are inoperable offered TAVI, therapy in patients with higher risks for sAVR should be determined by the Heart Team (Figure 1).
Figure 1. Management of severe aortic valve stenosis (Reproduced with permission of Oxford University Press on behalf of the European Society of Cardiology. © The European Society of Cardiology 2017. All rights reserved.). [3]
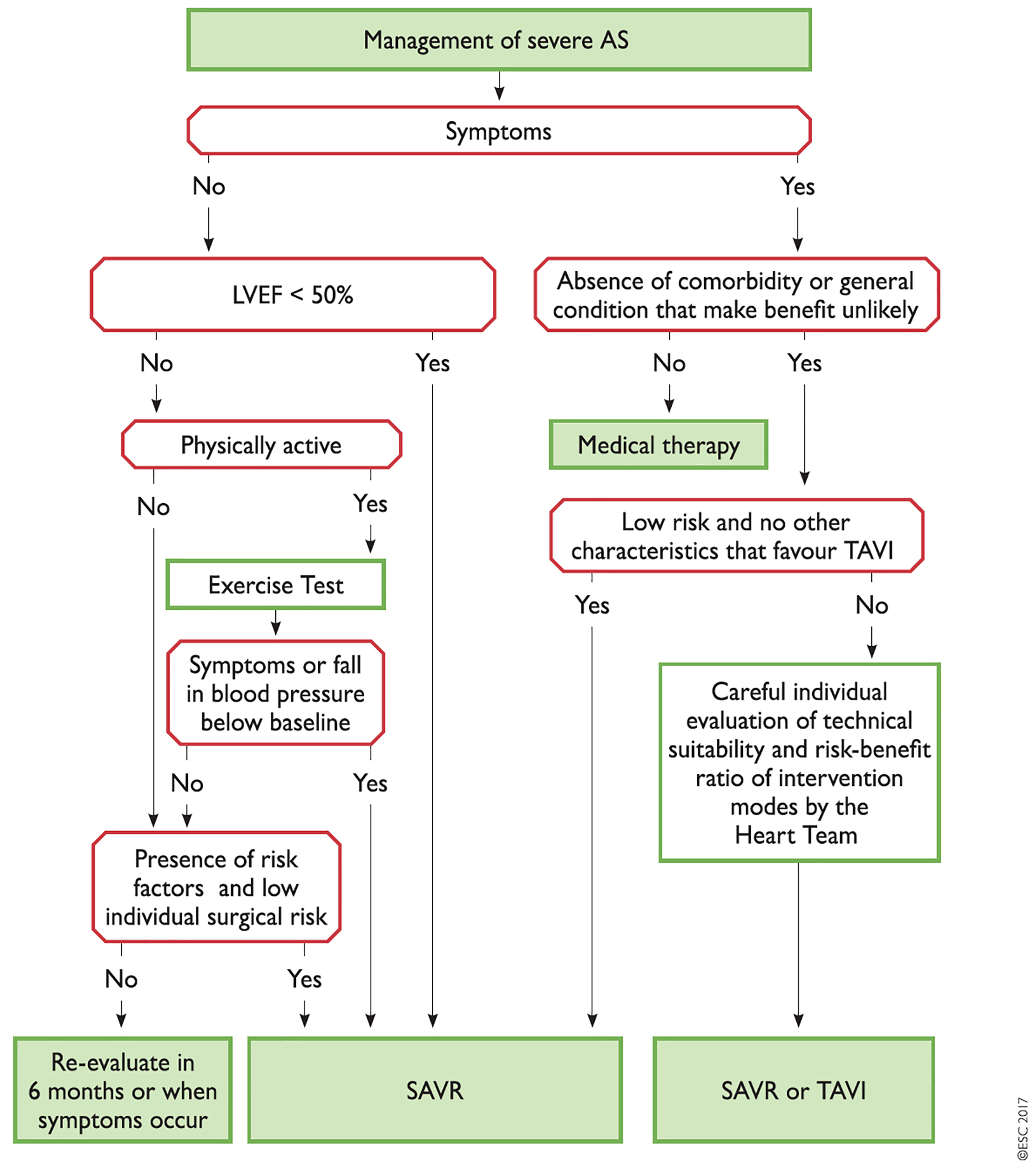
Members of the Heart Team need to work in a structured and collaborative way with each other and should be co-located at so-called “Heart Valve Centres”, centres of excellence for diagnostics and treatment of valvular heart disease. Discussions on patients with AS should focus on patient clinical characteristics, anatomical/technical aspects and cardiac comorbidities, identified during their assessment. Results from all three areas need to be taken into account and weighed against each other according to their importance in individual patients (Figure 2). In this context, Heart Teams also need to reflect on the specific strengths of sAVR and TAVI. While TAVI offers particular benefits in terms of reducing trauma and improving post-interventional mobilisation, sAVR has a particular strength when it comes to dealing with certain aortic valve/root anatomies such as asymmetric calcifications, low coronary origins or bicuspid aortic valves. It is also important to recognise that certain cardiac comorbidities, such as mitral regurgitation and tricuspid regurgitation, and also severe coronary artery disease, can be addressed at the time of surgery, while they have been identified as impairing outcomes after TAVI. As the durability of THVs is currently unproven, a patient age <75 years is seen as being less suitable for TAVI.
Figure 2. Aspects to be considered by the Heart Team for the decision between sAVR and TAVI in patients with severe aortic stenosis and increased surgical risk (Reproduced with permission of Oxford University Press on behalf of the European Society of Cardiology. © The European Society of Cardiology 2017. All rights reserved). [3]
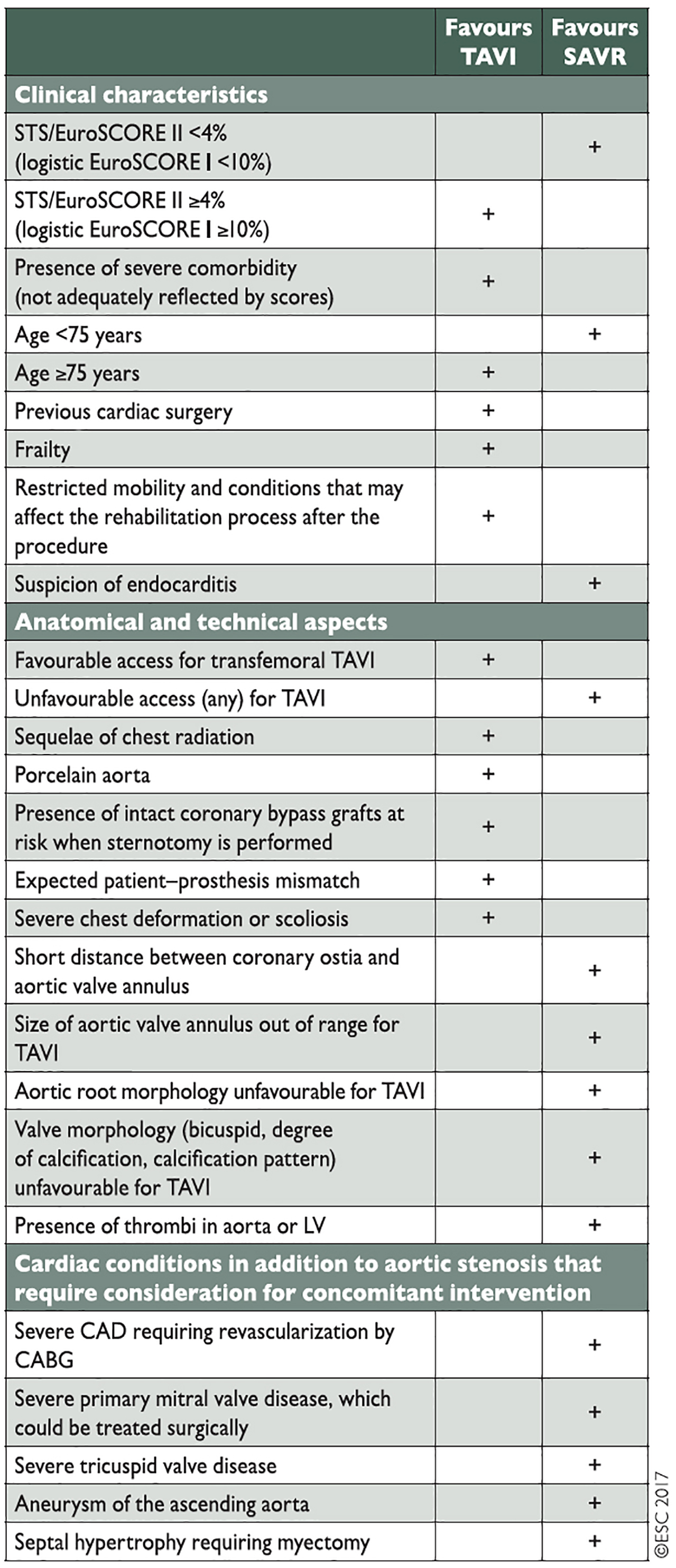
While it is important that Heart Teams take the available scientific experience as well as recommendations from guidelines into account when deciding on an individual patient’s therapy, they also need to reflect on centre-specific outcomes of the various treatment options such as TAVI and sAVR. A treatment strategy that may work very well in one centre may not be appropriate in another one.
Which clinical environment is needed to provide excellent heart valve therapy?
Heart Teams need to be able to work in an environment that provides comprehensive diagnostics and treatment options. “Heart Valve Centres”, in which a multidisciplinary team works together on a regular basis and as a result has established appropriate communication structures, seem to be ideally placed to become centres of excellence for the treatment of valvular heart disease in the future. In terms of treatment, surgical and interventional procedures such as valve repair, sAVR, aortic root surgery and TAVI should be routine. For an adequate assessment before treatment, it is paramount that these centres provide a comprehensive diagnostic armamentarium of the highest quality. This should include 3D, stress and perioperative transoesophageal echocardiography, cardiac computed tomography (CT), cardiac magnetic resonance imaging and positron emission tomography CT.
These centres need to be embedded into a network within the community and other referring hospitals. Networking between the various organisations and levels of care that build a cardiovascular network needs to be supported by communication structures which allow appropriate information exchange between interventional/non-interventional cardiologists, cardiac surgeons and also physicians from primary care.
Outcomes from a cardiovascular network need to be regularly assessed and available for various kinds of surgical and non-surgical interventions. These data should not only be used to support Heart Team discussions on the most appropriate treatment for patients with AS, but also submitted to national and European databases for further analysis.
Discussion
While surgical treatment for patients with AS has further matured over recent years, TAVI has made remarkable progress since it was started in 2004. The recent European guidelines for the treatment of valvular heart disease have recognised the large variety of treatment options available for patients and placed the Heart Team at the centre of the decision-making process to select individualised therapies for patients. However, the obvious question is how these guidelines can be implemented in the daily routine of large healthcare organisations.
Several years ago, we established at King’s College Hospital so-called multidisciplinary outpatient clinics. In these clinics, patients would be seen by cardiologists and surgeons together and their cardiac imaging optimised. In most patients this would allow an adequate early assessment, so that individualised patient pathways could be determined early, to save resources and time.
If patients were identified as treatable, their diagnostic tests would be completed, including multimodality cardiac imaging of a controlled standard. Those patients who were identified as higher risk for sAVR would be discussed at disease-specialised multidisciplinary meetings (aortic and mitral valve meetings), with the Heart Team and other specialists (e.g., anaesthetists, intensivists) present as needed.
Based on the comprehensive treatment options available at King’s, patients would not only be able to benefit from state-of-the-art surgical but also interventional treatment, using various THVs and access routes for TAVI.
Local outcome data for TAVI and sAVR are collected on a quarterly basis and support the decision-making process of the Heart Team, and also submitted for the mandatory national outcome registries.
Using this structure, we have been able to develop a Heart Valve Centre of excellence over recent years. It also allows training of young cardiac specialists in new surgical and interventional techniques using the most recent devices and keeps the focus of the entire team on optimised outcomes for patients.
Conclusions
The burden of valvular heart disease in general and AS in particular is steadily increasing. The recent developments in the treatment of AS in terms of less invasive surgical strategies and TAVI, and also new surgical and transcatheter devices, make it feasible to tailor aortic valve therapy to individual patient needs. Heart Teams should take the opportunity to work closely together despite historical differences and animosities between specialities, to evaluate these therapies and to identify the most appropriate treatment options for patients. As a consequence, they will not only provide optimal care, but also develop centres of excellence for the treatment of valvular heart disease, where innovative therapies can be further developed and the future generation of heart valve specialists effectively trained.
Sponsored by:




 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.