Introduction
Despite modern percutaneous coronary intervention (PCI) and coronary artery bypass graft (CABG) techniques, a significant percentage of stable coronary artery disease (SCAD) patients will continue to experience or will develop recurrent angina symptoms. Several randomised studies and meta-analyses [1-3] have shown that approximately 30% of patients revascularised for SCAD continue to experience angina symptoms, regardless of the procedure (PCI or CABG). Thus, the use of antianginal drugs represents a common treatment in those patients. Current ESC guidelines suggest the use of first- and second-line classes of drugs for the management of stable angina [4]; however, these patients often, if not always, have several concomitant risk factors or comorbidities which, on the one hand, alter the therapeutic approach and, on the other hand, may have in practice led to the development of their coronary artery disease. In this article, we are going to summarise the evidence in stable angina treatment recommendations in order to individualise patients’ treatment according to their particular characteristics and comorbidities.
Stable angina treatment in specific conditions
Stable angina and blood pressure levels
Current ESC guidelines on the treatment of stable angina pectoris [4] recommend the use of renin-angiotensin system (RAS) blockers since they may favourably alter prognosis, as well as the use of calcium channel blockers (CCBs), beta-blockers and long-acting nitrates for symptom relief. We have to bear in mind, however, that RAS blockers, as well as CCBs and beta-blockers, represent agents with significant antihypertensive effects. In effect, these agents are four of the five antihypertensive drug classes proposed by the current ESH/ESC guidelines for the management of arterial hypertension [5]. Thus, in patients with stable angina in need of antihypertensive treatment, ESH/ESC guidelines also suggest the use of these agents since, in addition to blood pressure (BP) decrease, these drugs also present other auxiliary properties (in terms of prognosis or symptom relief).
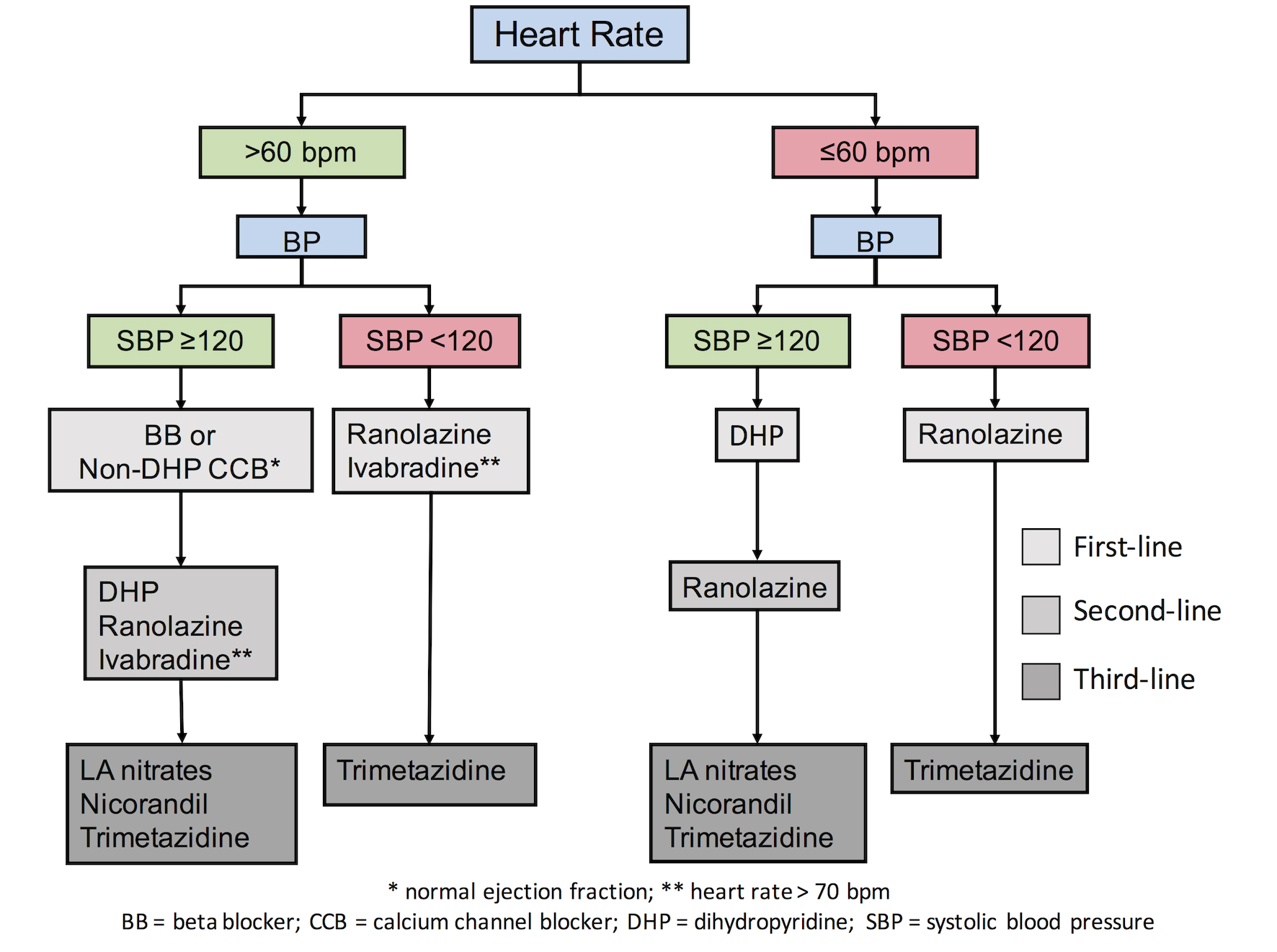
The question of the administration of these drugs is encountered however in patients with low BP levels. It is probable that low BP levels can provoke cardiovascular (CV) events in patients with stable angina, as explained principally by the J-curve phenomenon (i.e., an increased incidence of outcomes when the BP is markedly reduced). Despite the fact that there is no validated threshold of BP below which stable coronary heart disease (SCHD) patients may present adverse events, a threshold of 120 mmHg in systolic BP can be used as a reference. Recently, data from 22,672 patients with SCAD in the CLARIFY registry [6] revealed that patients with systolic BP/diastolic BP (SBP/DBP) of less than 120/70 mmHg had increased risk for CV events (adjusted HR 1.56, 95% CI: 1.36-1.81/adjusted HR 1.41, 95% CI: 1.24-1.61, respectively). In addition, an increased incidence of myocardial infarction for systolic BP reduced to less than 120-130 mmHg has been repeatedly reported in patients with a history of cardiac disease [7]. In the SPRINT trial [8], BP reduction to <120 mmHg was associated with an increase of multiple side effects such as hypotension, syncope, electrolyte abnormalities and acute kidney injury, while there was no benefit in terms of MI or cardiac events. Recently, an algorithm was proposed [9], according to which patients with stable angina and SBPs <120 mmHg should refrain from BP-lowering antianginal drugs in order to avoid excessive BP reductions (Figure 1). The authors instead proposed the use of drugs that do not affect (or minimally affect) BP levels in patients with lower SBP levels (<120 mmHg).
Figure 1. Individualised treatment according to patients’ comorbidities and risk factors [9].
Stable angina and heart rate levels
There is a robust body of evidence suggesting that increased heart rate (HR) in patients with coronary artery disease is deleterious, since it increases myocardial oxygen demand leading to ischaemia and anginal symptoms. Current ESC guidelines [4] recommend the use of heart rate-lowering agents such as beta-blockers, ivabradine and non-dihydropyridine (non-DHP) CCBs in order to decrease HR. We have to bear in mind, however, that excessive HR reduction may not only be deleterious because of the chronotropic incompetence-related symptoms and effects, but may also even increase the incidence of atrial fibrillation. After the results of the SIGNIFY study [10], which showed an increased risk for CV events and atrial fibrillation in patients with an excessive decrease of HR, a debate emerged on the threshold below which heart rate-lowering agents must not be used. Recently, a threshold level of 60 bpm (except for ivabradine which should not be initiated in HRs lower than 70 bpm according to the SIGNIFY findings) was proposed [9]. In patients with heart rates below this threshold, the use of drugs with minimal or no HR effect is advised (Figure 1).
Stable angina treatment in specific diseases
Current ESC guidelines on the management of stable angina suggest the use of several drugs for symptom relief, acknowledging however that none of these drugs may improve prognosis [4]. In addition, it seems that first- and second-line antianginal drugs are supported by the same level of evidence [4,9]. Thus, their use in specific conditions where patients can take advantage of their auxiliary effects beyond symptom relief should be considered.
Diabetic patients with stable angina
Approximately 33% of patients with stable CAD also suffer from diabetes mellitus (DM) [6]. The presence of DM leads to more extensive vascular disease and a more severe ischaemic burden (both anginal and silent) [4,9]. When treating diabetic patients with angina, drugs that have a positive or at least a neutral metabolic profile have to be preferred. Ranolazine is an antianginal drug with favourable effects in reduction of HbA1c levels [11]. In a randomised placebo controlled trial, the use of this agent was associated with a significant decrease in HbA1c levels, while the proportion of subjects achieving an HbA1c <7.0% was greater in the ranolazine arm compared to placebo (25.6% vs. 41.2%; p=0.0004). The use of beta-blockers in diabetics has been debated given the higher incidence of new onset DM or worsening of the glycaemic profile in these patients [5]. It seems that these unfavourable effects are limited to the majority of the non-vasodilating beta-blockers. In effect, vasodilating beta-blockers present a favourable metabolic profile since they improve insulin sensitivity and they do not cause deleterious effects on lipid profile [12]. Moreover, there are some data supporting the use of trimetazidine in diabetic patients. The administration of this drug (20 mg t.i.d. for two weeks) in a randomised placebo controlled trial resulted in a decrease in the fasting glucose plasma levels [13]; however, this study as well as the majority of studies with trimetazidine had a small sample size.
Therefore, agents such as ranolazine or vasodilating beta-blockers with their favourable metabolic profile, or agents such as ivabradine, nicorandil, CCBs and probably trimetazidine with their neutral profile, should be preferred in patients with angina and DM for symptom relief.
Stable angina and left ventricular systolic dysfunction
Approximately 70% of heart failure (HF) cases with reduced ejection fraction are directly linked to CAD, and in patients with HF and stable angina it is preferable to administer drugs that not only reduce angina attacks but may also have favourable prognostic effects. Administration of beta-blockers in patients not only reduces angina symptoms but may also delay the progress of HF while at the same time decreasing the rate of hospitalisations for HF and improving prognosis [4,14]. Moreover, the use of ivabradine in such patients is beneficial not only in terms of symptom relief but also in terms of reduction of hospitalisation for HF and improvement in prognosis in general [4,14]. In the BEAUTIFUL trial [15], administration of ivabradine in patients with left ventricular ejection fraction (LVEF) <40% resulted in a significant decrease in the composite endpoint of fatal and non-fatal myocardial infarction by 36% (p=0.001) and the need for revascularisation by 30% (p=0.016) in patients with an HR >70 bpm. Therefore, the use of beta-blockers and/or ivabradine in patients with stable angina and HF with a reduced ejection fraction is preferred since, along with symptom reduction, this has favourable effects in reducing CV morbidity and mortality [4,14]. On the other hand, the use of hydralazine/isosorbide dinitrate instead of the traditional renin-angiotensin-aldosterone system inhibition may be problematic since this combination may elicit angina attacks. Likewise, the safety of ranolazine in patients with HF with reduced EF (HFrEF) is uncertain and thus it has to be used with caution [14]. Nitrates may have a potential role, combining vasodilating and antianginal effects [14]. The administration of nicorandil and dihydropyridine (DHP) CCBs has also been shown to be safe in patients with HF and left ventricular systolic dysfunction (LVSD) [14]. Unfortunately, there are no significant data regarding the mid or preserved left ventricular ejection fraction (LVEF) range of HF patients (LVEF 40-49% and >50%). No treatment has yet been shown, convincingly, to reduce morbidity or mortality. Thus, in these patients we can use antianginal drugs that also have beneficial effects on their comorbidities.
Stable angina and atrial fibrillation
Atrial fibrillation may aggravate angina symptoms since it increases heart rate and thus myocardial oxygen consumption. Hence, in patients with stable angina and atrial fibrillation, heart rate-lowering antianginal drugs such as beta-blockers and non-DHP CCBs should be preferred. These drugs are useful not only for acute HR control but also for long-term control. HR-lowering agents with antianginal effects such as ivabradine are not suggested, since this drug is ineffective in AF [4,14]. Moreover, ivabradine in the SIGNIFY trial increased the incidence of atrial fibrillation compared to placebo (5.3% vs. 3.8%, p<0.001) [10], while a meta-analysis of 21,571 patients that assessed data from 11 studies with ivabradine showed that treatment with this agent was associated with an increased relative risk of AF of 1.15 (95% CI: 1.07-1.24, p=0.0027) [16]. Ranolazine seems to suppress atrial fibrillation and supraventricular arrhythmias in general [17,18]. In a retrospective study that enrolled 393 patients undergoing CABG, ranolazine (1,500 mg preoperatively followed by 1,000 mg twice daily for 10 to 14 days) was superior to amiodarone (400 mg preoperatively followed by 200 mg twice daily for 10 to 14 days) for the prevention of atrial fibrillation after CABG (17.5% vs. 26.5%, p=0.035) [17]. Moreover, in a phase 2 trial that evaluated the effects of ranolazine and dronedarone alone or in combination in patients with paroxysmal atrial fibrillation, the combination of ranolazine 750 mg b.i.d and low doses of dronedarone 225 mg b.i.d resulted in significant reductions of atrial fibrillation events when compared to placebo [18].
Possible combinations of antianginal drugs
Usually, patients with stable angina need more than one drug to suppress angina symptoms. Thus, in the majority of studies various antianginal drugs were administered on top of other antianginal drugs [4,10]. However, not all antianginal drugs can be combined. Combining ivabradine, ranolazine and nicorandil is not recommended because of the unknown safety profile [14]. In effect, there are no studies, or very few studies with small sample sizes, addressing this issue. Moreover, after the results of the SIGNIFY trial [10], the co-administration of ivabradine with non-DHP CCBs is contraindicated since it resulted in a significant HR reduction. In effect, verapamil or diltiazem are moderate CYP3A4 inhibitors and ivabradine is metabolised by CYP3A4. CYP3A4 inhibitors and inducers are prone to interactions with ivabradine and influence its metabolism and pharmacokinetics to a clinically significant extent [19].
Conclusions
Patients with stable angina usually have several comorbidities. An individualised treatment taking into account the various conditions and comorbidities should be proposed, since all antianginal drugs have roughly the same level of efficacy and there is no measurable survival benefit.




 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.