Introduction
A growing interest in sports activities raises concerns regarding the safety of exercise in athletes with various cardiovascular anomalies. In recreational sports, the incidence of sudden cardiac death and cardiovascular comorbidities is higher than expected and may even increase as more and older individuals participate in organised sports [1]. The risk of unrecognised symptomatic or asymptomatic left ventricle (LV) remodelling with subsequent heart failure (HF) in athletes increases with age [2,3]. Moreover, a greater number of patients with a history of cancer treated with cardiotoxic therapy seek participation in sports [4]. Equipped with an improved diagnostic and treatment armamentarium, and faced with a growing number of “at risk” participants, sports cardiologists will be increasingly faced with the dilemma of qualifying this population for exercise.
According to the well-known mortality classifications, hypertrophic cardiomyopathy is the leading cause of sudden cardiac death in athletes. In addition, HF of various other aetiologies can also contribute to cardiovascular fatalities in this population. Dilated cardiomyopathy is the main cause of HF in athletes, while acute myocarditis is the most frequent cause of acquired dilated cardiomyopathy in young athletes [5]. The latter may run an asymptomatic course and present with normal resting electrocardiography in up to 32% of those affected [6]. The inflammatory process of the myocardium can result in a fulminant HF in athletes engaged in intensive exercise, overtraining, doping, or drug abuse [7]. A recent registry analysis demonstrates that myocarditis can be a dominant cause of sudden cardiac death in athletes under 35 [8]. Hence, a detailed screening of athletes with high risk of HF by means of advanced diagnostic techniques is called for.
Heart failure in athletes – pathophysiology, inducing factors and clinical consequences
1. Exercise haemodynamics
A normal response to incremental dynamic exercise is a doubling in heart rate (HR), a threefold or fourfold increase in cardiac output (CO), a moderate increase in mean arterial pressure (MAP) and markedly decreased total peripheral resistance. This adaptive physiology is needed to meet the greatly increased demand for oxygen by a large mass of muscles which is perfused up to a 2.5 L·kg-1·min-1, or 100-fold above the basal values [9]! In untrained athletes, the mean stroke volume (SV) increases by one third before reaching a plateau, whereas in highly trained athletes the SV continues to rise progressively to the point of maximal exercise. Beta-adrenergic stimulation and improved diastolic compliance account for increased cardiac contractility and filling of up to 60% (Frank-Starling mechanism). Due to greatly increased heart rate, the improved diastolic filling is offset by a progressively shortened diastolic filling time. Because of these physical limitations, the maximally performing exercising heart can account for about half of the volume throughput measured in high-performance athletes, known to be in the region of over 30 L/min [10]. The source of “missing” blood volume - a long-standing dispute among exercise physiologists - is supposedly supplied by a “muscle pump”, a mechanism by which the blood is returned to the heart by the contracting muscles. However, the effectiveness of muscle pump in dynamic exercise has also been questioned. In view of these inconsistencies, a new, “evolutionary-biological” circulation model has recently been proposed in which the flow of blood is regulated by the metabolic demands of the tissues and the heart acts as an impedance pump which maintains pressure by rhythmically interrupting the flow [11,12]. Hence, the impairment of LV function concomitant with various deleterious factors influencing peripheral flow and tissue metabolism (cocaine, anabolics, amphetamine, exercise-induced hyperthermia), may not only affect the maximal exercise performance in athletes, but also contribute to serious cardiovascular complications during or after exercise.
2. From athlete’s heart to heart failure
A. Physiologic versus pathologic hypertrophy
The cardiomyocytes stop increasing in number soon after birth but retain marked plasticity which enables them to adapt to a variety of environmental stimuli with increase in size, or contraction. The heart possesses a unique three-dimensional architecture in which helically arranged myocardial fibres are embedded in a dense fibrous matrix [11]. Normal response to aerobic conditioning leads to enlargement of the myocytes with preserved architecture and corresponding expansion of the capillary beds. This “physiologic” hypertrophy is characterised by normal cardiac function (systolic and diastolic) and is paralleled with an increase in the body muscle mass. In pathological hypertrophy on the other hand, the myocyte growth occurs in response to haemodynamic load and a variety of neurohumoral mediators in which the capacity of the capillary beds fails to supply adequate amounts of nutrients and oxygen. It leads to negative remodelling, fibrosis, cell death and cardiac dysfunction/heart failure.
B. Causes of cardiac hypertrophy
In addition to various hormones (growth hormone, insulin, insulin-like growth factor, thyroxine) the mechanical forces transmitted to the heart by the flowing blood during intensive exercise are known to be largely responsible for signalling which leads to cardiac enlargement in athletes. The myocytes contain a number of mechanotransducers which respond to and convert rheological stimuli to biochemical events. These biochemical sensors have been identified chiefly as proteins at the level of the sarcomere, the intercalated disc and the sarcolemma. They act as triggers for adaptive or maladaptive remodelling [13]. Unlike the skeletal muscle which is built for strength of contraction, the myocardium is primarily built to withstand the stretch. This is confirmed by the significantly higher myocardial fibre resting tension and by the presence of the above-mentioned network of supporting fibrous matrix, surrounding individual and groups of myocardial fibres; epimysium, perimysium and endomysial weave [11].
C. Concentric and eccentric hypertrophy
From the perspective of geometry, physiological hypertrophy can be classified into concentric and eccentric. In concentric hypertrophy, known to occur in individuals engaged in sports with a high static component such as weightlifting or wrestling, the heart is subject to bursts of high pressure, with only a moderate increase in cardiac output and oxygen consumption. Repeated bouts of pressure overload cause increased thickness of the LV free wall and of the septum, and result in reduced dimensions of the LV cavity. On the other hand, in exercise with a predominantly dynamic component, such as long distance running or cross-country skiing, the total body demand for oxygen is far greater than in “static” sports. The heart in turn handles large volume throughputs at only a moderate increase in mean pressure. Sustained large volume loads lead to a global enlargement of the heart and its cavities (Figure 1).It should be noted that the physiological adaptive response to exercise leading to increased heart dimensions presents a continuum which overlaps with pathological hypertrophy. It is up to the clinician to decide where this boundary may be for a particular individual.
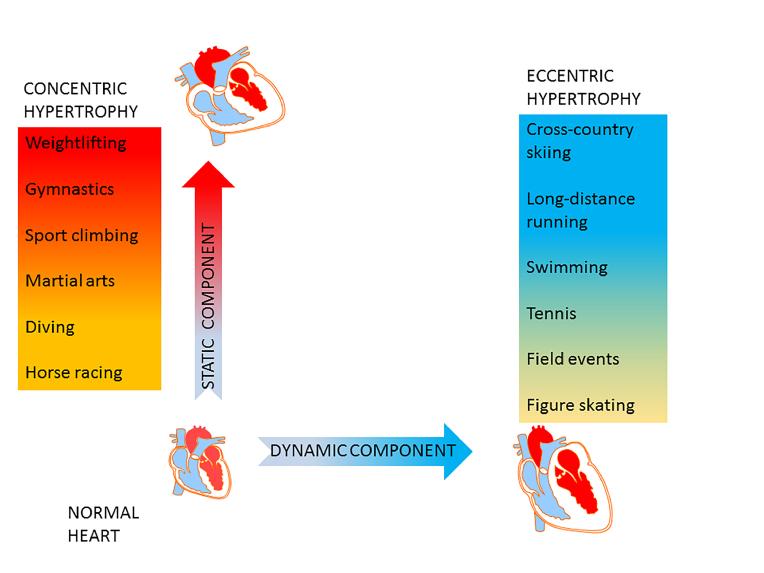
Figure 1. Cardiovascular adaptation to exercise.
Exercise with a predominantly static component is characterised by sustained periods of increased mean arterial pressure and peripheral resistance, and only a moderate increase in oxygen consumption. Physiological response to elevated mean pressure is concentric left ventricular wall thickening with relative contraction of the chamber volume (red panel). Dynamic exercise is typically associated with a marked increase in oxygen consumption and cardiac output, a moderate increase in mean arterial pressure and a marked drop in peripheral resistance. Such a volume load results in eccentric left ventricular enlargement (blue panel).
D. Factors determining development of heart failure in athletes
As mentioned, extreme physical effort can precipitate acute HF, or exacerbate a pre-existing ventricular dysfunction in athletes with unrecognised myocardial damage induced by various pathological processes (acute myocarditis, drug- or doping-induced cardiac cell damage, post-myocardial infarction dysfunction, severe valve defect, uncontrolled arterial hypertension, toxic process or past medical interventions, such as chemotherapy or radiotherapy) (Table 1). High static sports can lead to development of hypertrophic cardiomyopathy and, in turn, to chronic diastolic and/or systolic HF. Intensive exercise can induce reversible heart dysfunction, which in some cases becomes persistent and leads to “pro-arrhythmogenesis” [14]. Severe athletic training, known for release of pro-inflammatory cytokines from the exercising muscles, has been implicated in the pathophysiology of chronic HF and may be a factor in the unrecognised dysfunctional heart in athletes [15]. Furthermore, the coexisting inflammatory process in a distant organ or system, such as respiratory tract infection or untreated post-exercise trauma, may negatively affect the ultrastructure of the heart and contribute to ominous arrhythmic events [16].
Mental stress and exercise-induced hyperactivity of the sympathetic nervous system during sport competition can cause a massive release of catecholamines into the circulation, which of themselves can exacerbate myocardial dysfunction and cause acute HF in predisposed athletes (age, diabetes, chronic ischaemic heart disease, hyperthermia, dehydration and electrolyte imbalance, chronic infection or inflammatory process). Finally, the tragic aftermath of unrecognised HF in athletes is exercise-induced, lethal ventricular arrhythmia.
Symptoms of heart failure in athletes – diagnostic management
Clinical manifestation of chronic HF in athletes as well as non-athletes can be non-specific, underestimated and/or misdiagnosed, with possibly dire consequences. Thus, athletes suffering from HF may be asymptomatic or present with atypical symptoms. Moreover, athlete candidates can present with structural and functional cardiac abnormalities, which are precursors of HF (Figure 2). Ambitious athletes in particular are inclined to dissimulate symptoms or blame them on non-cardiac causes. The most challenging group are elderly athletes who often attribute their exertional dyspnoea or fatigue to ageing. Diagnosis and appropriate management of chronic HF in this group can in addition be compounded by other comorbidities such as COPD, diabetes mellitus or depression. Moreover, the fatigue or drop in performance is often ignored and attributed to overtraining. It should be noted that electrocardiography, basic laboratory tests, and biomarkers (N-terminal proBNP, BNP), can be falsely negative in some groups of athletes suffering from HF. Hence, the persistence of symptoms in cases with normal physical examination should always be supplemented by cardiac imaging, prolonged electrocardiographic monitoring (Holter-ECG) and cardiopulmonary exercise test (CPET).
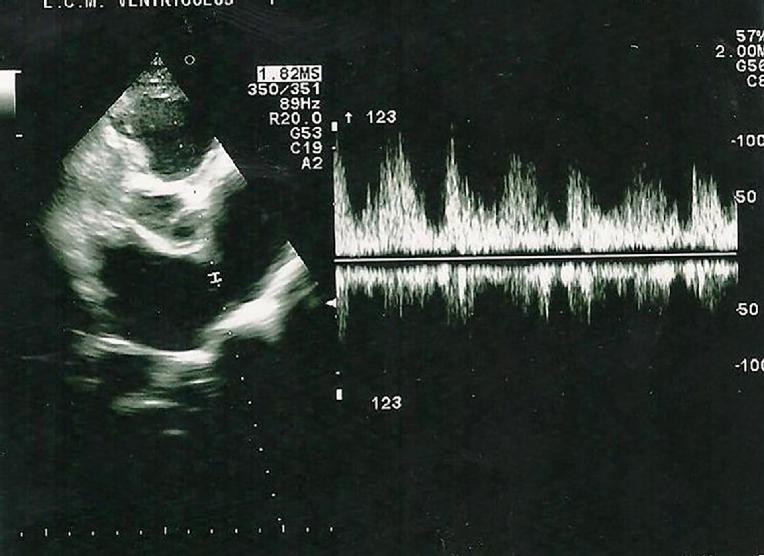
Figure 2. An example of an athlete candidate presenting with structural and functional cardiac abnormalities which are precursors of HF.
A 21-year old female athlete candidate without a history of cardiovascular disease or heart failure symptoms. Transthoracic echocardiography: ASD type II (width 14 mm) with left-to-right shunt in PW conventional Doppler, significantly enlarged right ventricle and right atrium, normal mean pulmonary artery pressure, TAPSE and regional tricuspid annulus velocities. (See also Skalik R [18]. Figures 3,4,5,6)
Recommended sequential diagnostic process in athletes with symptoms of heart failure should include:
- Basic laboratory tests: blood morphology, CRP, TSH, troponin, CK-MB, D-dimers, electrolytes, arterial blood gases
- Chest X-ray, spirometry, resting electrocardiography (ECG)
- Transthoracic echocardiography (TTE) and, if inconclusive, transoesophageal echo (TEE)
- Holter – ECG, ambulatory blood pressure monitoring
- CPET for diagnosis of detraining or overtraining, assessment of aerobic performance in athletes with HF for treatment strategy and prognostication
- Cardiac magnetic resonance imaging (CMR) for differentiation between ischaemic and non-ischaemic cardiomyopathy
- Genetic tests; hereditary dilated cardiomyopathy, hypertrophic cardiomyopathy, arrhythmogenic dysplasia of right ventricle
The following symptoms may indicate heart failure in athletes and should alert sports cardiologists to consider advanced imaging such as echocardiography or CMR:
- Shortness of breath, unexpected drop in performance, dyspnoea on exertion
- Persistent fatigue and muscle pains of uncertain aetiology, refractory to anti-inflammatory medications, physiotherapy, reduction in training intensity or exercise cessation
- Mental disorder – especially when combined with fatigue
- Pre-syncope or dizziness
- Persistent cough
- Nocturia or oliguria - in elderly athletes
- Lower extremities swelling
- Anginal pains - in elderly athletes
- Recent history of respiratory tract infection and drop in performance
- Persistent heartbeat irregularities
Systolic HF of various origins is one of the most frequent causes of persistent exercise intolerance in athletes. Echocardiography is a basic tool to diagnose systolic or diastolic HF in athletes, differentiate and quantify LV hypertrophy. As mentioned, intensive exercise in healthy athletes can result in physiologic LV hypertrophy that is always accompanied by well preserved ventricular systolic and diastolic function. Pathologic left ventricle hypertrophy on the other hand can result in diastolic LV dysfunction and culminate in diastolic and/or systolic HF. In some cases, the physiologic LV hypertrophy can overlap with the onset of pathologic remodelling and thickening of the cardiac muscle. Hence, a precise and timely diagnosis of the pathologic hypertrophy can not only prevent HF, and possibly sudden cardiac death, but can direct to the appropriate therapy.
Pathologic hypertrophy of the left ventricle in athletes can be suspected when:
- Thickness of interventricular septum or any other LV segment exceeds 12-13 mm and is refractory to detraining (LV wall thickness may reach 15 mm in healthy endurance athletes, but LV global and regional diastolic function remain preserved)
- Septal/posterior wall thickness ratio is above 1.3
- Global and/or regional diastolic LV function is impaired (conventional Doppler: E/A <1, IVRT >100 ms; mitral annulus tissue Doppler: E’/A’ <1, E’<8 cm/s)
In borderline cases, strain imaging and/or post-exercise evaluation of the regional diastolic LV function with use of tissue Doppler echocardiography can be performed (healthy athlete: normal LV longitudinal strain at rest, amelioration of regional diastolic LV function in response to exercise; increase in mitral annulus E’ velocity, increase in mitral annulus E’/A’ ratio) [17].
Diastolic dysfunction and/or HF can develop in athletes suffering from uncontrolled arterial hypertension, hypertrophic cardiomyopathy or with a history of anabolics abuse. Diastolic HF can be suspected in symptomatic athletes who present with a drop in exercise performance or shortness of breath in association with the following findings: LV hypertrophy (usually above 12-13 mm), global LV diastolic dysfunction (impaired LV relaxation, pseudonormalisation of LV diastolic filling, or restrictive LV filling pattern), elevated LV filling pressure (E/E’ >15), left atrial (LA) enlargement (LA volume index above 40 ml/m2), preserved LV ejection fraction (HFpEF). However, enlargement of the LA can also be observed in healthy endurance athletes. Therefore, tissue Doppler diastolic parameters and strain rate can be decisive for the final diagnosis [18]. In controversial cases (asymptomatic or poorly symptomatic athletes with LV hypertrophy, E/E’ between 8 and 15), the measurement of NT-proBNP, BNP (NT-proBNP >220 pg/ml or BNP >200 pg/ml) or global diastolic LV parameters (E/A <0.5, DT >280 ms) can be helpful. CPET can also be used for differentiation between the pathologic LV hypertrophy and physiologic exercise-induced LV hypertrophy (maximal oxygen consumption above 50 ml/kg/min is more consistent with athlete’s heart). Complicating the issue is the fact that clinical symptoms of diastolic HF can also be observed in overtrained or detrained healthy athletes. Hence, echocardiographic screening of athletes with unexplained exercise intolerance and athletes in the high-risk group (antihypertensive therapy, familial history of arterial hypertension or heart failure) should be mandatory.
2D echocardiography can be normal or inconclusive in some athletes with a history of acute myocarditis and ischaemic heart diesease. In such cases, CMR or computed tomography may be the best tool to establish a correct diagnosis. In addition, CMR can be helpful in the diagnosis of HF in athletes with:
- metabolic cardiomyopathies (sarcoidosis, haemochromatosis, amyloidosis- speckle tracking and strain imaging are also advisable)
- congenital heart defects (sinus venosus ASD, non-compaction cardiomyopathy)
Causes of heart failure in athletes
Hereditary dilated cardiomyopathy (HDCM)
Dilated cardiomyopathy (hereditary or secondary to acute viral myocarditis) is the leading cause of systolic HF in athletes. However, congenital/acquired heart defects or toxic factors (drugs, anabolics) can induce dilated cardiomyopathy in this population. The diagnostic process of HDCM can be especially difficult in athletes participating in endurance sports because adaptive changes in the heart muscle are similar to those found in HDCM. Moreover, dilated cardiomyopathy may not produce a significant drop in aerobic capacity on CPET in some athletes [19].
Basic diagnostic tools: echocardiography and CMR (additionally, genetic tests and familial history of the disease can be helpful).
Echocardiography
- dilatation of heart cavities with regional contractility abnormalities (lowered ejection fraction; LVEF <50%), abnormal geometry of left ventricle with relative mitral and/or tricuspid valve regurgitation
- abnormal LV global, regional systolic and/or diastolic function as measured by conventional and tissue Doppler echocardiography (abnormal E/A, S’, E’/A’, E/E’, MAPSE, TAPSE)
- abnormal longitudinal strain (also an important prognosticator in athletes with
- HDCM)
- lack of reduction in left ventricle dimensions in response to exercise
- cessation
- exercise echocardiography: lack of increase in LVEF or decrease in LVEF in
- response to exercise (indicative of non-physiologic dilatation of left ventricle)
CMR
- differentiation between hereditary and ischaemic/inflammatory dilated cardiomyopathy or myocardial damage
Dilated cardiomyopathy versus athlete’s heart on echocardiography
In ambiguous cases, the following echocardiographic signs point to dilated cardiomyopathy:
- abnormal global and/or regional systolic and diastolic LV function on conventional and tissue Doppler echocardiography, abnormal longitudinal strain
- lowered LVEF with regional contractility abnormalities
- lack of reduction in heart cavity dimensions after detraining
- familial history of HDCM
Table 1. Causes of heart failure in athletes.
|
Causes |
Clinical notes and diagnostic tools* |
|---|---|
|
Myocarditis |
Mainly athletes below 35 years, viral or rarely parasitic origin, sometimes subclinical; echocardiography, CMR |
|
Arterial hypertension |
High static and high dynamic sports can contribute to hypertensive diastolic and/or systolic HF; echocardiography, CMR |
|
Drug abuse or doping |
Cocaine or amphetamine addiction, anabolics abuse; echocardiography, CMR |
|
Iatrogenic myocardial damage |
Chemotherapy (anthracyclines), radiotherapy (chest irradiation) – dose-dependent; echocardiography, CMR |
|
Inherited dilated cardiomyopathy
|
Genetically conditioned (e.g., deletion in dystrophin gene); echocardiography, CMR |
|
Ischaemic cardiomyopathy |
Post-myocardial infarction contractile dysfunction with lowered EF (below 50%); echocardiography, CMR |
|
Hypertrophic cardiomyopathy |
Diastolic and/or systolic HF; echocardiography, CMR, genetic tests |
|
Left ventricle non-compaction (LVNC) |
Genetically conditioned, differential diagnosis with exercise-induced hypertrabeculations in athletes; echocardiography, CMR – the most reliable diagnostic tool |
|
Acquired or congenital valve defects |
Mitral or aortic insufficiency, aortic stenosis, VSD, ASD, Ebstein anomaly; echocardiography, CMR |
|
Pulmonary thromboembolism |
Right ventricular failure following posttraumatic DVT in high-risk/high-intensity sports ; echocardiography (McConnell sign, reduced TAPSE and systolic tricuspid annulus velocities, enlargement of right heart, diastolic flattening of interventricular septum, pulmonary hypertension), computed tomography |
* See also Skalik R. Qualifying athletes for exercise. E-Journal of ESC Council For Cardiology Practice. 2014;12:1-11.
Acute myocarditis and dilated cardiomyopathy (DCM)
Unrecognised acute myocarditis in athletes with asymptomatic or poorly symptomatic clinical course of the disease can culminate in acute or chronic HF, especially in athletes continuing intensive physical effort. The exercise increases sympathetic activation and oxygen consumption which can precipitate the development of HF in athletes with active myocarditis. Hence, athletes with a history of recent respiratory tract infection, drug abuse or doping with persistent clinical symptoms (cough, shortness of breath, fatigue, heartbeat irregularities) should undergo detailed medical investigation including electrocardiography (ECG), biomarkers (troponin, CK-MB, NT-proBNP), echocardiography or CMR and CPET. It is also advisable that asymptomatic athletes after recent respiratory tract infection should undergo medical check-up (detailed physical examination, resting ECG - negative T-waves, pathological Q-waves, intraventricular conduction disturbances can indicate acute myocarditis even in athletes with normal echocardiogram) before they resume intensive physical training. It must be stressed that some patients with normal LV systolic function after acute myocarditis can develop symptomatic diastolic HF over time [20]. Hence, athletes with a history of acute myocarditis should be regularly screened for symptoms of HF, diastolic global and regional LV dysfunction on echocardiography.
Echocardiography
- mild myocarditis: regional contractility of the left ventricle can be normal, slight pericardial effusion or local hyperechogenicity is sometimes observed
- severe or moderate myocarditis: a typical picture of dilated cardiomyopathy or varying severity of regional contractile LV/RV dysfunction, abnormal regional systolic function on tissue Doppler echocardiography (reduced S’, MAPSE <12 mm, TAPSE <16 mm)
- abnormal LV/RV global and regional diastolic function as measured by conventional or tissue Doppler echocardiography (abnormal E/A, mitral annulus E’/A’ and E/E’)
- abnormal longitudinal or circumferential strain rate (also an important prognosticator)
CMR
- differentiation between inflammatory and ischaemic LV dysfunction
Chemotherapy-/radiotherapy-induced heart failure in athletes
There is increasing evidence that higher levels of physical activity are associated with a reduced risk of cancer recurrence, and a longer survival after a cancer diagnosis. However, many cancer patients undergo potentially cardiotoxic chemo and/or radiotherapy that can induce cardiac damage and subsequently HF years after the termination of effective cancer therapy. Hence, recovered cancer patients wishing to participate in moderate- to high-intensity sports should be regularly screened for heart dysfunction by means of echocardiography. Moreover, subclinical LV dysfunction on strain imaging can precede contractile impairment and symptomatic HF in athletes after cancer treatment. It has been estimated that, after successful chemotherapy, up to 50% of cancer patients will present with heart dysfunction within the next 10-20 years after treatment, and 5% of them will suffer from chronic HF and incidents of malicious arrhythmias. In many cases, heart dysfunction or HF after cancer treatment is asymptomatic.
The symptomatic or asymptomatic athletes with a history of cancer treatment may suffer from heart dysfunction or HF if the following abnormalities are found:
- New ST segment abnormalities, pathological Q-waves, ventricular or supraventricular arrhythmia on ECG or Holter ECG
- Increased NT-proBNP or BNP level
- Global and regional systolic or diastolic dysfunction on echocardiography (conventional or tissue Doppler echocardiography, strain imaging, 2D speckle tracking)
- Global or regional cardiac dysfunction on CMR
Conclusions
Extreme physical effort is a source of cardiovascular pressure and volume overload that can cause acute heart failure or exacerbate pre-existing ventricular dysfunction in athletes with previously unrecognised myocardial damage. Some athletes suffering from heart failure may not present with typical symptoms of the disease. Hence, diagnostic cardiovascular screening with the use of advanced imaging tools may be necessary in symptomatic, but also some asymptomatic athletes with increased risk for heart failure participating in high-intensity sports.



 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.