Summary
The aim of this case report is to present the first experience with a newly utilised echocardiographic contrast agent in a patient with hypertrophic obstructive cardiomyopathy treated with PTSMA. The interventional group has a vast experience with the use of Levovist® for this procedure, which has been shown to have excellent efficacy and safety in PTSMA. Since Levovist® is no more available, the authors have tested Gelafundin®, a colloidosmotic volume-expander consisting of Gelatine-Polysuccinate, in comparison to Levovist® in the same patient.
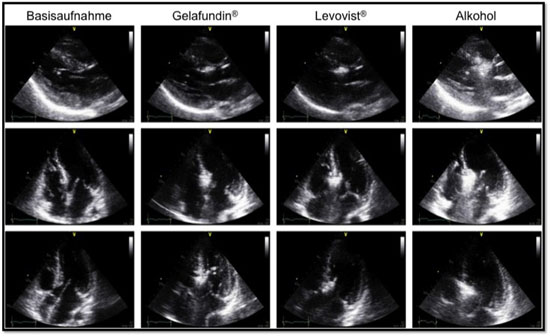
Figure. Comparison between Gelafundin® and Levovist® in the same patient during PTSMA.
Levovist® injected after recording of echocardiographic images acquired with injection of Gelafundin® resulted in no further enhancement of the already contrasted basal septal area (Figure). Moreover, there was no further opacification of other myocardial areas apart from those already indicated by Gelafundin®. Gelafundin® remained long enough in view, so that there was enough time to evaluate all echocardiographic views for possible misplacements. Most importantly, there was no arrhythmogenicity during or after Gelafundin® injection.
The authors’ conclusion was that Gelafundin® seems safe and efficacious as a newly introduced intracoronary injected echocardiographic contrast agent for PTSMA.
Comments
The presentation by Pfeiffer B, et al. is of great interest, because it proposes a possible solution to a significant problem. Myocardial contrast echocardiography is sine qua non for PTSMA and the choice of the specific contrast agent may have a tremendous impact on the efficacy and safety of the procedure. The ideal contrast agent should offer good contrast imaging with clear pigmentation of the target area, slow capillary runoff that ensures a stable demarcation of the target area, rapid washout (e.g. destruction of bubbles with increased echo gain) in case of possible misplacement, no allergic potential and of course no arhythmogenicity. Levovist® has shown most of these properties, so as to be regarded as the ideal contrast agent for PTSMA. According to own experience, the use of agitated radiographic contrast produces low quality opacification of the target area, with the possibility to miss a possible misplacement, while Sonovue®, a widely used via the intravenous route contrast agent seems to have a very fast runoff when used in the coronary arteries rendering a safe and detailed scrutiny of the contrasted areas extremely doubtful.
References
- Sigwart U. Non-surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. Lancet 1995;346:211-4.
- Seggewiss H. Percutaneous transluminal septal myocardial ablation: a new treatment for hypertrophic obstructive cardiomyopathy. Eur Heart J 2000;21:704-7.
- Seggewiss H. Current status of alcohol septal ablation for patients with hypertrophic cardiomyopathy. Curr Cardiol Rep 2001;3:160-6.
- Singh M, Edwards WD, Holmes DR, Jr., Tajil AJ, Nishimura RA. Anatomy of the first septal perforating artery: a study with implications for ablation therapy for hypertrophic cardiomyopathy. Mayo Clin Proc 2001;76:799-802.
- Rigopoulos A, Sepp R, Palinkas A, Ungi I, Kremastinos DT, Seggewiss H. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: collateral vessel communication between septal branches. Int J Cardiol 2006;113:e67-9.
- Faber L, Seggewiss H, Gleichmann U. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: results with respect to intraprocedural myocardial contrast echocardiography. Circulation 1998;98:2415-21.
- Rigopoulos AG, Seggewiss H. A decade of percutaneous septal ablation in hypertrophic cardiomyopathy. Circ J 2010;75:28-37.
- Faber L, Seggewiss H, Welge D, et al. Echo-guided percutaneous septal ablation for symptomatic hypertrophic obstructive cardiomyopathy: 7 years of experience. Eur J Echocardiogr 2004;5:347-55.
- Seggewiss H, Faber L. Percutaneous septal ablation for hypertrophic cardiomyopathy and mid-ventricular obstruction. Eur J Echocardiogr 2000;1:277-80.
- Faber L, Welge D, Hering D, et al. Percutaneous septal ablation after unsuccessful surgical myectomy for patients with hypertrophic obstructive cardiomyopathy. Clin Res Cardiol 2008.
- Veselka J, Prochazkova S, Duchonova R, et al. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: Lower alcohol dose reduces size of infarction and has comparable hemodynamic and clinical outcome. Catheter Cardiovasc Interv 2004;63:231-5.
- Faber L, Welge D, Fassbender D, Schmidt HK, Horstkotte D, Seggewiss H. One-year follow-up of percutaneous septal ablation for symptomatic hypertrophic obstructive cardiomyopathy in 312 patients: predictors of hemodynamic and clinical response. Clin Res Cardiol 2007;96:864-73.
- Veselka J, Zemanek D, Tomasov P, Homolova S, Adlova R, Tesar D. Complications of low-dose, echo-guided alcohol septal ablation. Catheter Cardiovasc Interv 2010;75:546-50.
Notes to editor
Presented by: Dr. Barbara Pfeiffer, Dr. Angelos Rigopoulos, Prof. Hubert Seggewiss
Medizinische Klinik , Leopoldina-Krankenhaus Schweinfurt Germany
The content of this article reflects the personal opinion of the author/s and is not necessarily the official position of the European Society of Cardiology.


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.