It is pertinent to ask what do we actually mean by polarity?
Within a single cell, the answer might be the ability to distinguish top from bottom or front from back (Figure 1).
In epithelia there is clearly a top and bottom, as there is an apical compartment that forms one side of the cell layer and a basolateral compartment that forms the other. From the need to actively create and isolate the apical compartment it would seem that the basolateral compartment is the default (reviewed in Bryant and Mostov, 2008; Pilot and Lecuit, 2005). In addition to the apical-basal specification of cell polarity, polarity within the plane of the epithelium has been described. Planar cell polarity (PCP) was originally described in fruit flies, but has now been shown to be a feature of vertebrate epithelia and some mesenchymal tissues.
Planar cell polarity defines the proximal (front) and distal (back) regions of cells and allows cells to form organised arrays, such as is seen in the cuticle of the wing and thorax, and ommatidia of the eyes, in fruit fly (reviewed in Simons and Mlodzik, 2008; Zallen, 2007). The signalling pathways that regulate the establishment and maintenance of both apical-basal polarity (ABP) and PCP have been elucidated and are highly conserved through metazoan evolution (Goldstein and Macara, 2007; Zallen, 2007; Figure 2). Furthermore, the PCP and apical-basal systems seem to be linked, as Scrib is a key molecule in both pathways.
Recently, primary cilia have been implicated in cell polarisation. These are microtubule-based membrane extensions occurring on the apical surface of many cells which derive from the inherited centriole. Increasingly they are being seen as a specialised apical compartment, playing roles both in sonic hedgehog signalling and PCP signalling (reviewed in Gerdes et al, 2009). Thus, it has been suggested that the primary cilium may be a super-specialised apical compartment in vertebrate cells. In keeping with this, it has been suggested that PCP proteins in higher vertebrates are closely corralled into the apical compartment within the primary cilium, which may act like a signalling antennae on the cell (Angers and Moon 2009). With these basic cellular concepts in mind we can turn to the development of the heart and ask to what extent is polarity an important determinant in heart development, either in the cells that contribute to the heart or in the fully differentiated cardiac cell.
Polarity and formation of the early heart
We now are aware of a number of distinct cell populations that contribute to the developing heart. The primitive heart tube is composed of an endothelial cell tube surrounded by a single layer of cardiomyocytes. Disruption of a number of key genes known to be essential for the establishment and/or maintenance of ABP, including Pals1 and protein kinase Ciota (PKCi, a member of the family of atypical protein kinase C (aPKC); Figure 2) have been shown to result in marked abnormalities in the formation of the heart tube in the early zebrafish embryo, confirming the importance of this process for linear heart tube formation.
Specifically, PKCi and Pals1 are required for polarised organisation of the early epithelial heart tube and coherence of the myocardial cells during the heart cone stage of cardiac development in zebrafish. Later in development, the heart tube fails to elongate correctly and the myocardial cells do not expand in size (Rohr et al, 2006). Relatively few genes involved in establishment of ABP appear to have been knocked out in mouse. One exception, however, is Par3, which is a component of a complex of three proteins (Par3/Par6/aPKC), which play essential roles in the polarisation of epithelia, by regulating junctional structures (Macara et al, 2004). However, although Par3 is essential for epicardial formation, it seems to be dispensable for the formation of the early heart tube (Hirose et al, 2006). Thus, although it seems likely that ABP is important for early heart formation in the mouse as it is in teleosts, this has not yet been shown unequivocally.
The cells of the primitive heart tube are later joined by second heart field cells that take a similar origin, but whose route is altered by the folding of the embryo and the dissolution of the dorsal mesocardium which forces them to stream from below the pharynx to add on to either end of the formed heart tube. The second heart field continues to add on cardiomyocytes to the forming heart and augments the atria, the right ventricle and the outflow tract (reviewed in Moorman et al, 2007). Recent studies have shown that although the Isl1-expressing cells of the second heart field do not migrate as individuals, rapid proliferation within the caudal wall of the pericardium feeds cells into the arterial and venous poles of the heart (van den Berg et al, 2009).
Interestingly, expression of a dominant-negative form of the PCP effecter, Rho kinase, within the Isl1-expressing cells of the second heart field, results in malformations of both the venous and arterial poles of the heart (Hildreth et al, 2009 and currently unpublished data). This supports the idea that polarisation of the cells within the second heart field is a critical step in their movement into the poles of the heart.
Polarised cell migration into and within the developing heart
Neural crest cells are a multipotent population of cells that arise in the dorsal region of the neural tube and migrate extensively within the embryo to contribute to a variety of organs, including playing essential roles in outflow tract septation (reviewed in Hutson and Kirby, 2007). During migration, these cells adopt characteristic phenotypes with broad lamellipodia extending in the direction of movement at the front of the cell, and narrower tails at the rear of the cell where the cell detaches from the substrate allowing forward movement (Kuriyama and Mayor, 2008).
These specialised membrane structures are supported by the cytoskeleton of the cell and complex signalling networks regulate the rapid construction and dismantling of these structures as the cell migrates. Elegant studies from the group of Roberto Mayor have shown that non-canonical Wnt/PCP signalling, via the key effecters RhoA/Rho kinase and Rac, plays crucial roles in the directed migration of neural crest cells, at least in Xenopus and zebrafish embryos (De Calisto et al, 2005; Matthews et al, 2008; Carmona-Fontaine et al, 2008). Interestingly, in these systems, neural crest cells exhibit contact inhibition of migration, during which leading edge cells at the front of the migratory field extend and collapse their lamellipodia in response to contact with other neural crest cells. Consequently, the cells change direction in response to this contact, resulting in overall forward movement of the neural crest cell group. In contrast, contact with other cell types does not have this effect allowing them to infiltrate non-neural crest-derived tissues. Abrogation of PCP signalling disrupts this contact inhibition of migration, resulting in neural crest cells climbing over one another and little forward movement (Carmona-Fontaine et al, 2008).
Although this mechanism has been convincingly described in fish and frogs, there is no data as yet to support a similar role for PCP signalling in mammals or birds. Indeed, although mouse lines carrying mutations in key PCP genes develop cardiac defects (see below), there is no evidence that this is caused by disruption of neural crest cell migration into the outflow tract. Indeed, the neural crest cells appear to migrate normally into the outflow tract in both the loop-tail (Vangl2 mutant; Henderson et al, 2001) and circletail (Scrib mutant; Phillips et al, 2007) mouse mutants. Thus, whether PCP signalling is essential for directional migration of neural crest cells in higher vertebrates, and whether this is important for the development of the septated outflow tract, remains to be established.
Connexin 43 is the major gap junctional protein found in neural crest cells and has been implicated in the polarised cell movements required for their directional migration (Xu et al, 2006). Connexin 43 mutants have outflow tract malformations (Ewart et al, 1997; Sullivan et al, 1998), however, conditional deletion of connexin 43 from neural crest cells does not result in outflow abnormalities (Kretz et al, 2006), suggesting that it may not be an essential factor in the migration process. However, polarised behaviours may be important once NCC reach the outflow tract cushions and begin to condense. Mice with conditional inactivation of N-cadherin in neural crest cells have outflow tract alignment defects, typically double outlet right ventricle. The neural crest cells appear to migrate normally to the outflow tract, but once they reach the cushions they remain rounded and show abnormal adhesion to their neighbours. How this might disrupt outflow tract remodelling remains unclear, but the authors speculate that N-cadherin localised to the adherens junctions of condensing neural crest cells may be required for the propagation of cytoskeletal forces generated by their alignment and/or interaction, and that these forces may be required for the normal rotation of the outflow tract cushions (Luo et al, 2006).
Thus, polarisation of neural crest cells both as they migrate, and as they condense within the outflow cushions, might be essential for normal morphogenesis of the outflow tract.
The epicardium is a polarised tissue which forms a layer over the surface of the myocardium in the mature heart. It is also essential for heart development. During formation of the epicardium in mouse embryos, epithelial cysts detach from the proepicardium and reattach to the surface of the myocardium where they spread to form a monolayer over the surface of the heart. A subset of cells of the epicardial monolayer transforms into mesenchyme and migrates into the myocardium of the ventricular wall. These cells contribute endothelial and smooth muscle cells to the forming coronary vessels, fibroblasts that form the fibrous skeleton of the heart (reviewed in Olivey et al, 2004; Wessels and Perez-Pomares, 2004) and, controversially, have been suggested to contribute to cardiac muscle (Cai et al, 2008; Zhou et al, 2008).
Par3, a key ABP component, has been shown to play an important role in the formation of the mouse epicardium. In Par3 knockout embryos, there is disruption of the apical domain of proepicardial cells, which prohibits the formation of epithelial cysts and blocks formation of an intact epicardial monolayer. In the absence of the resulting epicardially-derived cells, the coronary vessel network fails to form and the ventricular myocardium remains thin (Hirose et al, 2006). Our recent studies have shown that the coronary vasculature forms but is severely disrupted in the planar cell polarity mutant loop-tail (Lp), in which the Vangl2 gene is disrupted (Phillips et al, 2008). In Lp/Lp mice, the epicardial cysts are able to attach to the heart but then epicardially-derived cells fail to migrate into the myocardium effectively, with the majority remaining on the surface of the heart (unlike in man, the coronary vessels are intra-mural in mouse). This results in a disorganised mass of tortuous vessels on the ventricular surface, frequently associated with hypoplasia of the ventricular wall. Perhaps surprisingly, Vangl2 is not expressed in the epicardium from which the cells that form the coronary vessels are derived, but is strongly expressed in the myocardium of the ventricles.
This suggests a non cell-autonomous role for Vangl2 in formation of the coronary vessels, perhaps caused by disruption of the cytoarchitecture of the cardiomyocytes within the ventricular myocardium (Phillips et al, 2008; see below).
Roles for PCP signalling in remodelling of the cardiac outflow tract
Once the cardiac chambers have formed, the heart septates and remodels to take on its mature form. One key septation/remodelling process involves the separation of the common outflow tract into the aorta and pulmonary trunk and the concomitant alignment of these vessels with their respective ventricular chambers.
This process has been shown to be dependent on PCP signalling. During this remodelling process, cardiomyocytes from the outflow tract wall migrate into the outflow cushions, resulting in their complete muscularisation and formation of the muscular outlet septum (van den Hoff et al, 1999).
As a result of the failure of cardiomyocytes to polarise and migrate, the outflow tract cushions remain unmuscularised in Lp/Lp fetuses lacking functional Vangl2 (Figure 3), although it is unclear whether this is sufficient to cause the double outlet right ventricle observed in this mutant (Henderson et al, 2001; Phillips et al, 2005). Similar abnormalities in the connections of the great arteries with the ventricular chambers are observed in several other mouse lines carrying mutations in other PCP proteins. For example, outflow tract malformations are observed in mice carrying mutations in Scrib (Phillips et al, 2007), Dishevelled 1-3 (Hamblet et al, 2002; Etheridge et al, 2008), Wnt11 (Zhou et al, 2007; Nagy et al, 2009) and Wnt5a (Schleiffarth et al, 2007), suggesting that this pathway may be fundamental to the normal septation and alignment of the great arteries with the ventricular chambers. Studies from the group of Sylvia Evans (Zhou et al, 2007) have elaborated a signalling pathway utilising canonical as well as non-canonical Wnt signalling, culminating in the expression of TGF2, which may regulate this polarisation process.
However, conditional inactivation of components of these pathways within the different cell types that make up the developing outflow vessel, will be essential in order to fully elaborate how these Wnt pathways participate in outflow tract development.
Cardiomyocyte polarisation and myocardial function
The process by which cardiomyocytes in the embryonic heart become polarised to form the rod-shaped cells of the adult myocardium remains poorly understood.
Until approximately E12.5 of mouse development, cardiomyocytes within the ventricular wall are approximately spherical and their junctional components are distributed throughout the cell membrane (Hirschy et al, 2006). By E13.5, the cardiomyocytes have begun to elongate slightly, appearing as bean-shaped cells, and cell-junction proteins have become punctuate within the membrane. Over the next five prenatal days, and the following two weeks of postnatal life, the cells gradually lengthen, until they eventually acquire the characteristics of adult cardiomyocytes, with the majority of the cell junction proteins localised to specialised intercalated discs which join the cells end to end. Specialised adhesion complexes along the lateral membranes (costameres) attach the rod-shaped cardiomyoctes to the surrounding extracellular substratum, and are responsible for transferring force (Figure 1).
The organisation of the cardiomyocytes within the mass of the ventricular wall is highly controversial. Some authors have suggested that cardiomyocytes are aggregated into secondary bundles or sheets, although more recent descriptions show that the myocytes themselves are aggregated together within a three-dimensional mesh (Pope et al, 2008; Lunkenheimer et al, 2006; reviewed recently in Henderson and Anderson, 2009), without any secondary structure.
Clonal lineage tracing studies have shown that the orientation of cardiomyocyte growth differs within different areas of the ventricle. Specifically, in different regions, the growth of the clones of cardiomyocytes follows a circumferential, radial or perpendicular orientation, relative to the ventricular wall (Meilhac et al, 2003, 2004). These clonal patterns thus correlate, at least in a general way, with the distribution of cells observed in the adult ventricle and begin to show how the complex architecture of the adult ventricular myocardium is achieved.
Studies of the ABP and PCP pathways are beginning to give hints about how polarisation of cardiomyocytes may become established, but the clues seem to be most evident at the very earliest stages of heart development. Although these studies are still in their infancy, this might relate to the epithelial nature of the primary heart tube, where each primitive cardiomyocyte is in contact with several other surrounding cardiomyocytes, and thus there is great potential for ABP and/or PCP signalling to occur.
This compares with the mature myocardium where cardiomyocytes attach to one another only at intercalated discs. For example, Scrib is the vertebrate homologue of the Drosophila scribble gene, which is a key component of the pathway that establishes ABP in fruit flies. This protein, although cytoplasmic, is a component of a complex that forms at the cell membrane and is required to establish normal cellular architecture (Figure 2). Mutations in scribble in Drosophila result in grossly disorganised epithelial structures within the larvae and enormous tissue overgrowth (Bilder et al, 2000). The general PCP-type phenotype when Scrib is disrupted, together with its interaction with the core PCP gene Vangl2 (Murdoch et al, 2003; Phillips et al, 2007) suggest that Scrib may link the processes of APB and PCP in the developing mammalian embryo.
Mutations of Scrib in the circletail (Crc) mouse result in a typical PCP phenotype; the embryos have severe neural tube defects resulting from disruption of convergent extension movements and abnormalities in the organisation of hair cells in the inner ear that are characteristic of disruption of PCP (Murdoch et al, 2003; Montcouquiol et al, 2003). These Crc mutants also have heart defects similar to those observed in other PCP mutants (Phillips et al, 2007) and the localisation of the PCP protein Vangl2 is disrupted in early cardiomyocytes, raising the possibility that these two proteins may be interacting in the developing myocardium (Phillips et al, 2007).
In mammalian systems, Scrib has been shown to be important in cell migration in several cell types including epithelial cells and tumour cells, localising to adherens junctions (Navarro et al, 2005; Nola et al, 2008). In Crc mouse mutants lacking functional Scrib, the heart appears abnormal at the linear heart tube stage, and closer examination has revealed that the cardiomyocytes are disorganised within the tube with abnormal distribution of key junctional proteins such as N-cadherin and -catenin. Later in development, the Crc/Crc embryos have marked defects in chamber development, with small, under-developed ventricles and poor formation of ventricular trabeculae.
This ultimately results in a defect reminiscent of non-compaction of the ventricular myocardium, an increasingly reported cardiomyopathy that can arise during fetal life (Breckenridge et al, 2007). Loop-tail mutants also have defects in the formation of the ventricular myocardium (Phillips et al, 2008), in addition to the abnormalities in the development of the outflow myocardium described earlier. Although early formation of the heart tube appears normal in Lp/Lp embryos, and indeed Vangl2 is not expressed in the myocardium until approximately E9.0 when the heart begins to loop, the myocardium is thinned in approximately 50% of Lp/Lp embryos by E13.5. Analysis of the developing cardiomyocytes at this stage has revealed that whereas the cells are beginning to elongate in control embryos, they remain spherical in Lp/Lp embryos.
This defect is likely mediated by Rho kinase, a key effecter of the PCP pathway, which is an important modulator of the cytoskeleton (Phillips et al, 2008). Support for the idea that PCP signalling may play a critical role during organisation of cardiomyocytes within the ventricular wall has come from analysis of mouse embryos lacking Wnt11 (Nagy et al, 2009). Analysis of the developing ventricle in Wnt11 null mice revealed that the cardiomyocytes appeared disorganised, and moreover, that the cell adhesion molecules N-cadherin and -catenin were misexpressed. These defects were accompanied by disruption of the cytoskeleton. All of these defects closely resemble those seen in Crc mice (Phillips et al, 2007), thus, PCP signalling may play important roles in the acquisition of polarity within developing cardiomyocytes and in their proper organisation within the myocardial wall.
The multiple embryonic abnormalities observed in PCP mutants, together with their perinatal death, precludes the analysis of the consequences of loss of PCP function in adult life. The production and analysis of conditional alleles of core genes within both the ABP and PCP pathways will thus be crucial for the elaboration of the importance of these pathways in cardiac development and in postnatal life. Although the role of the core ABP and PCP components have not been analysed in conditional deletion studies, the roles of some of their key effectors have been investigated. RhoA, cdc42 and Rac are important mediators of cell polarisation, localising to the front (cdc42 and Rac) or rear (RhoA and its mediator Rho kinase) of migrating cells (reviewed in Heasman and Ridley, 2008). Loss of RhoA and ROCK function from cardiomyocytes causes defects in the ventricular myocardium very similar to that seen in the loop-tail mutant (Phillips et al, 2008), suggesting that these effectors may lie downstream of PCP signalling in the myocardium.
Although the other small Rho GTPases have not been knocked out specifically in cardiomyocytes during development, they have been mis-expressed in the adult myocardium resulting in cardiac hypertrophy (reviewed in Loirand et al, 2006; Brown et al, 2006). Moreover, endothelial-specific deletion of Rac1 in mouse embryos resulted in almost compete abrogation of blood vessel formation and mid-gestation lethality (Tan et al, 2008), supporting essential roles for this polarity protein during embryogenesis. Thus, these factors may play essential roles, regulating the actin cytoskeleton, during both the acquisition and maintenance of cardiac cell polarity.
Figure Legends.
Figure 1. Polarisation of distinct cell types.
A) ABP is a feature of epithelial tissues. The apical surface contacts a lumen whereas the basal surface contacts the basement membrane. The junctional belt containing the tight and adherens junctions localises close to the apical surface and functionally separates the cell into apical and basolateral domains. Subcellular structures such as villi and cilia are found associated with the apical surface of the cell.
B) In migrating cells, lamella and lamellipodia are found at the proximal region (front) of the cell whereas a tail is found distally (at the rear).
C) Cardiomyocytes are found early in development as almost spherical cells, with their junctional components (blue) distributed throughout their membrane. Through the latter half of gestation, extending into the postnatal region, the cells gradually become rod-shaped, with their cell-cell junctional components (blue) found at the ends of the cells. Attachments to the extracellular matrix are found on their lateral surfaces (red). The process underlying this polarisation process is currently unknown.
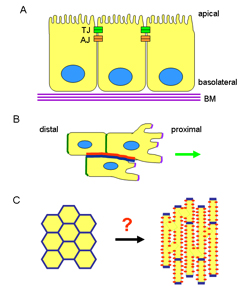
Figure 2. Key molecules involved in the establishment and maintenance of
A) apical basal polarity and B) planar cell polarity in Drosophila and the association between the two types of polarity (C).
A) Spatial segregation and functional antagonism between the Scribble and Par complexes is crucial for the polarisation of epithelia. In Drosophila, Scribble restricts the Par complex to the apical membrane whereas the Par complex recruits the Crumbs complex and together they exclude the Scribble complex from the apical membrane. These interactions keep the Par complex apical to the adherens junctions and Scribble basal to the adherens junctions. Similar functional antagonism is thought to take place in mammalian cells (modified from Humbert et al, 2006).
B) Planar cell polarity is established by the accumulation of core proteins in the proximal and distal regions of the cell. The transmembrane proteins Vangl2 (strabismus), Frizzled and Celsr1 (flamingo) are central to this. Frizzled, dishevelled and diego (dgo) localise to the distal region, whereas Vangl2 and prickle (pk) localise proximally. Celsr1 (flamingo) localises both proximally and distally. Recruitment of dishevelled activates a downstream signalling cascade which signals via the small GTPases RhoA and Rac, resulting in modification of the cytoskeleton. This ultimately results in polarisation of cells within epithelia and polarised cell migration (modified from Strutt, 2005; Jenny and Mlodzik, 2006). Scribble is a component of the ABP complexes in Drosophila but can also acts within the PCP pathways in mammals. Scrib might therefore link ABP with PCP.
C) Association between ABP and PCP within the plane of an epithelium.
A= apical, B=basolateral, D=distal, P=proximal, SAR= sub apical domain, SJ= septate junctions (correspond to tight junctions in vertebrates), ZA= zona adherens (corresponds to adherens junctions in vertebrates).
aPKC=atypical protein kinase C; bPIX=beta PIX, Dgo=diego, Dlg= discs large, Dvl=dishevelled, Fz=frizzled, Lgl=lethal giant larvae, p=phosphorylation, Pk=prickle, Scrib=scribble, Std=stardust; all other acronyms are standard usage.
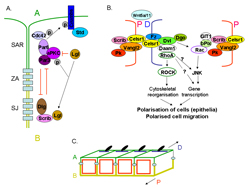
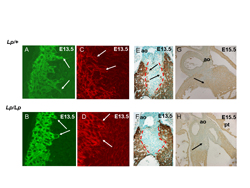
Figure 3. Abnormalities in cardiomyocyte polarity result in failure to muscularise the outlet septum in loop-tail (Lp) mice.
A,B) In control littermates stained with MF20 for cardiac muscle, cardiomyocytes from the outflow tract wall can be seen protruding into the outflow cushions at E13.5, clearly exhibiting membrane extensions at the front of the cells (arrows). In Lp/Lp littermates, the cells do not extend into the cushions.
C,D) At the same stage, visualisation of the filamentous actin within the cell with fluorescent phalloidin reveals that the cells exhibit stress fibres (arrows in C) in control hearts , whereas the actin appears cortical (arrows in D) in Lp/Lp littermates.
E,F) At E13.5, the outflow tract cushions are unstained in control hearts (blue region bounded in red) although cardiomyocytes are protruding into the tissue (arrows in E). The non-muscularised region of the outflow cushions is already broader in Lp/Lp embryos at E13.5 (F).
G,H) By E15.5, the outlet septum is completely muscularised (stained brown) in control embryos (arrow in G), whereas it remains non-muscularised in Lp/Lp hearts (arrow in H).
Ao=aorta.
Conclusion:
In summary, an understanding of the role of cell polarity in the developing heart is important not only with regard to understanding how normal developmental processes might be disturbed and lead to congenital heart malformation, but also for understanding the aetiology and progression of acquired heart disease. There are also important questions to be answered about how immature heart cells become polarised and take on their adult form; this will be important for cardiac regeneration programmes where immature cardiac cells need to be induced to develop rapidly in order to restore cardiac function.
The study of conditional “knock out” alleles of key polarity genes is likely to be essential if we are to fully realise the importance of cell and tissue polarity in all of these processes.


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.