BARCELONA, Spain – Saturday 30 August: Stimulating the vagus nerve, which regulates the body’s internal organ systems including the heart, does not improve cardiac function in heart failure patients, according to results of the NEural Cardiac TherApy foR Heart Failure (NECTAR-HF) trial.
NECTAR-HF was presented as a Hot Line today at ESC Congress 2014, with simultaneous publication in the European Heart Journal.
The trial’s failure to show a cardiac benefit of vagal nerve stimulation (VNS) was unexpected, said study investigator Faiez Zannad, MD, PhD, from l'Institut Lorrain du Coeur et des Vaisseaux Louis Mathieu, in Vandoeuvre-lès-Nancy, France.
“There is robust pre-clinical data showing the benefit of VNS, but the NECTAR-HF trial failed to demonstrate a successful clinical translation of this protocol,” he said.
The study is the first randomised, controlled trial designed to evaluate the safety and efficacy of right-sided VNS.
It enrolled 96 heart failure patients from 24 centres across Western Europe to assess the impact of six months of VNS on cardiac function, as well as on cardiac biomarkers, exercise capacity, and quality of life.
Patients, who were about 59 years old and receiving optimal medical therapy, had the VNS device implanted in their neck, near the right vagus nerve, and connected to a pulse generator implanted under the skin of the chest. 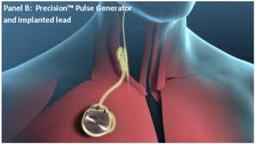
After baseline testing of the system, patients were then randomized to either a control group in which the devices remained switched off, or an active VNS group in which the mean stimulation amplitude was 1.24 mA at the start of the study, and 1.42 mA at the 3-month follow-up visit.
After six months, the study found no difference in objective endpoints between the groups.
Both groups had comparable changes from baseline in the primary endpoint of left ventricular end-systolic dimension (LVESD), as well secondary echocardiographic endpoints, exercise capacity, and levels of the heart failure serum biomarker N-terminal prohormone brain natriuretic peptide (NT-proBNP).
However, there were significant differences between the groups for three subjective endpoints related to quality of life.
Analyses of the Minnesota Living with Heart Failure Questionnaire (MLHFQ) and the Short Form 36 Health Survey (SF-36) demonstrated statistically significant improvement in quality of life with VNS treatment compared to controls (MLHFQ p=0.049; SF-36 Physical and Mental, p=0.02 and 0.24, respectively).
Additionally, 62% of patients in the VNS group had an improvement of at least one point from baseline in their New York Heart Association (NYHA) functional classification compared to only 45% of control patients (p=0.032).
“The safety profile for this application of VNS appeared acceptable, with an overall infection rate of 7.4%, which is comparable to that in patients implanted with a VNS system for the treatment of epilepsy,” noted Professor Zannad.
Given that preliminary studies suggested a benefit of VNS, there are a number of possible explanations for NECTAR-HF’s negative findings.
First, there is still much to learn about the appropriate dosing and technique of VNS. Data from epilepsy VNS studies show that higher-amplitude dosing might be more effective, but it is often not possible because it causes patient discomfort, said Professor Zannad.
Second, patients in the NECTAR-HF trial were relatively well-managed on medical therapy alone, making them perhaps not the best candidates to show a strong benefit of VNS.
Third, the six-month study period may have been too short to detect changes in cardiac function.
Although patients were blinded to their group assignment, meaning they were not told if they were receiving active treatment, it is possible that those in the active treatment group were able to feel the sensation of stimulation. If this was the case, patients could have been vulnerable to a placebo effect, knowing that they were receiving active therapy.
For this reason, the higher quality of life scores among VNS patients compared to controls should be interpreted with caution, he said.

 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.