I - Atherosclerosis and endothelial dysfunction
- Atherosclerosis is a systemic inflammatory disease. It is:
- The leading cause of death in Europe
- Propagated by a genetic predisposition
- Also propagated by risk factors, such as cigarette smoking, arterial hypertension, diabetes mellitus, and hypercholesterolemia.
- The disease is initiated by continuous damage to the endothelium from the aforementioned risk factors, which leads to the development of endothelial dysfunction 1.
Inflammation is an important pace maker of atherogenesis2, which involves the diseased cells of the vessel wall such as endothelial cells and smooth muscle cells and circulating inflammatory cells. Adhesion and invasion of T cells and monocytes leads to an accelerated inflammatory reaction in the vessel wall and enhances progression of atherosclerosis.
Besides these processes, several other effects such as lipid deposition and pathological proliferation and migration of smooth muscle cells contribute to the atherosclerotic process. Destabilisation of plaques with subsequent plaque rupture initiates a cascade of pro-coagulatory events and may lead to the occlusion of the diseased vessel and to cardiovascular events such as myocardial infarction and stroke.
II - Periodontitis and vascular disease
Periodontitis is thought to be caused by local infection with periodontal pathogens and subsequent local inflammation.
a) The risk factors for periodontis are similar to those of atherosclerotic disease
Abnormal host responses contribute to a more rapid disease progression in some patients. The prevalence of periodontal disease is high and this disease is more common with cigarette smoking, obesity, and diabetes mellitus – risk factors that also predispose to atherosclerotic disease.
b) There is an association between the presence of periodontitis and coronary artery disease and an increased cardiovascular event rate
Epidemiological studies demonstrated an association between the presence of periodontitis and coronary artery disease and an increased cardiovascular event rate, respectively 3-7. This association appears to depend on the severity of periodontal disease but is independent of the presence of traditional cardiovascular risk factors. However, other studies did not find significant associations after adjustment for important confounders 7.
c) Periodontitis causes endothelial dysfunction together with systemic inflammation
The degree of chronic inflammation in the oral cavity during periodontitis is sufficient to induce systemic inflammatory reactions by stimulation of humoral and cell-mediated pathways 6,9-11.
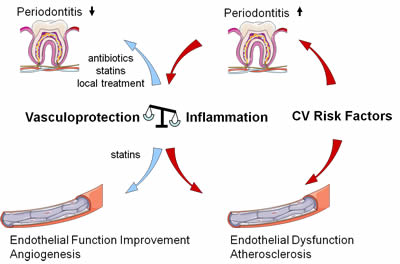
As outlined above, inflammatory processes are crucial in the pathogenesis of endothelial dysfunction and atherosclerosis. Indeed, recent clinical investigations have demonstrated that severe periodontitis causes endothelial dysfunction together with systemic inflammation.
d) Endothelial function can be improved after successful periodontal treatment
Importantly, endothelial function was improved after successful periodontal treatment 12-15. In patients without atherosclerotic disease, severe periodontitis was associated with an increased intima/media thickness of the common carotid arteries, a surrogate parameter of early atherogenesis and in patients with essential hypertension, an association between severity of periodontitis and left ventricular mass was recently suggested 9,16.
e) A correlation was demonstrated between chronic periodontitis and incidence of coronary heart disease
In a current publication by Dietrich et al., the association between periodontitis and risk of coronary heart disease was further investigated in 1203 men of the VA Normative Aging and Dental Longitudinal Studies 17. These men were followed up with triennial medical and dental examinations for up to 35 years. A clear correlation was demonstrated between chronic periodontitis in these initially healthy men with incidence of coronary heart disease, independent of established cardiovascular risk factors. Interestingly, this close association was only present in men <60 years of age.
f) The mechanisms leading to vascular damage by periodontal disease are less clear
The mechanisms leading to vascular damage by periodontal disease are less clear. Periodontal pathogens seem to play an important role in this context. Antibodies to these pathogens are associated with coronary artery disease, the periodontal pathogen burden correlates with the presence of coronary artery disease, and bacterial DNA was found in atherosclerotic plaques of coronary arteries 18-22.
Oral and intravenous infection with the periodontal pathogen Porphyromonas gingivalis (P.g.) accelerated atherosclerosis in apolipoprotein E-deficient (ApoE-/-) mice, an established mouse model of atherosclerosis, and in normocholesterolemic and hypercholesterolemic pigs 23-26. Infection of human aortic endothelial cells with P.g. induced an up-regulation of the adhesion molecules VCAM-1, ICAM-1, and E-selectin and of the pro-inflammatory cytokines interleukin-6 and interleukin-8 27.
In addition, it was demonstrated that P.g.-induced pathologies were mediated through activation of toll-like receptors TLR-2 and TLR-4, receptors of the innate immune system that seem to be involved in the pathogenesis of atherosclerosis 28-33.
III - Clinical Implications
a) Act on etiologic factors
Periodontitis shares several common etiologic factors e.g. smoking, diabetes, hypertension but also low social class and unfavourable health care with coronary heart disease (CHD). In the VA Normative Aging and Dental Longitudinal Studies mentioned above 1200 men were followed for up to 35 years and give clear evidence for an association between chronic periodontitis and the risk of coronary heart disease independent of classical risk factors 17.
However, until today, no convincing evidence has been presented demonstrating a true causal relation between periodontitis and vascular disease. There is a definite need for prospective studies assessing periodontal disease and clinical endpoints of cardiovascular disease.
b) Patients at risk for atherosclerotic disease especially need regular dental care and statins, perhaps.
Until this data is present, health care providers should keep in mind that poor oral health and the presence of periodontitis may negatively affect the blood vessel wall. Regular dental care with treatment of periodontitis using local dental and antibiotic therapy 34 seems advisable in patients at risk for atherosclerotic disease. Interestingly, first data suggests that patients on statin medication exhibit fewer signs of periodontal inflammatory injury than subjects without statin therapy 35.
Figure : Periodontis and Atherosclerosis : Process and treatment
The content of this article reflects the personal opinion of the author/s and is not necessarily the official position of the European Society of Cardiology.



 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.