Introduction
Spontaneous coronary artery dissection (SCAD) is a rare non-atherosclerotic cause of acute coronary syndromes (ACS). More cases are now being identified due to increased awareness and earlier use of invasive angiography in patients presenting with acute chest pain.
It is important to recognise SCAD, as patient characteristics and management differ substantially from typical ACS cases. SCAD patients are often younger, and more likely to be female without the classic cardiovascular risk factors. Results of revascularisation with percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) are suboptimal. Conservative management is the preferred option with spontaneous healing of the dissection in the majority of uncomplicated cases.
Pathophysiology
SCAD is caused by sudden disruption of the coronary artery wall, resulting in separation of the inner intimal lining from the outer vessel wall. The trigger is thought to be either an intimal tear or bleeding from the vasa vasorum, resulting in intramural haematoma. Pressure-driven expansion of the haematoma causes propagation of the dissection plane with formation of a true lumen, and a thrombus containing a false lumen.
SCAD should be distinguished from dissection caused by plaque rupture in patients with atherosclerosis or catheter-induced iatrogenic dissections. Patients with SCAD have fragile arterial walls with no atheroma or calcification to limit propagation of dissection. Therefore, cases with SCAD will often have more extensive dissections, and non-affected coronary artery segments appear smooth and disease-free on angiography.
Epidemiology
SCAD is estimated to be responsible for 0.1% to 0.4% of all ACS cases. [1] It is an important cause of ACS in young women, responsible for up to 25% of all ACS cases in women <50 years of age. [2]
The Mayo Clinic registry of 87 consecutive patients with SCAD reported a mean age of 43 years; 82% were female. [3] No cause was identified in 45% of all cases, highlighting that many cases of SCAD remain unexplained. The commonest identified predisposing factors were postpartum, fibromuscular dysplasia (FMD), connective tissue disease and hormonal therapy. Potential stressors include extreme physical exertion, particularly in young male patients, intense emotional stress, sympathomimetic drugs (such as cocaine, amphetamines), childbirth and Valsalva-like activities (such as coughing, retching, vomiting). Triggers for SCAD are thought to increase shear stress on the coronary artery wall, often mediated by elevated catecholamine levels and intra-abdominal pressure. [4]
Clinical presentation and diagnosis
ST-elevation myocardial infarction (MI) is present in up to 50% of patients presenting with SCAD, with non-ST-elevation MI in the rest. Most patients with SCAD will have an elevated troponin level. Life-threatening ventricular arrhythmias and sudden cardiac death are recognised early complications.
The diagnosis of SCAD is made at the time of coronary angiography. Findings can be graded into three types.
Type 1
The classic description is of a longitudinal filling defect, representing the radiolucent intimal flap. There is often contrast staining of the arterial wall with appearance of a double lumen (Figure 1).
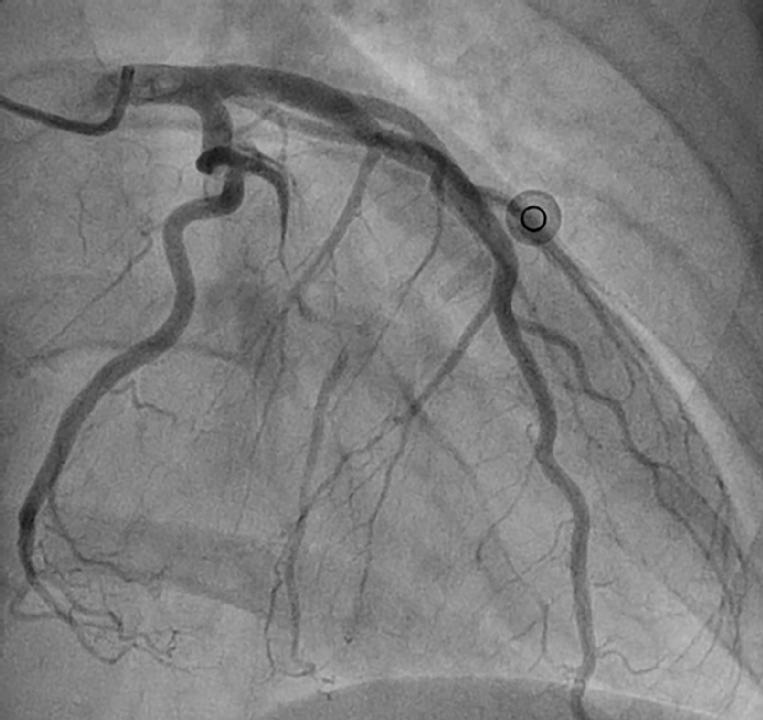
Figure 1. Coronary angiogram image from a 47-year-old female admitted with chest pain, dynamic inferior ST-elevation and raised troponins. The AP projection shows Type 1 SCAD involving a large proximal obtuse marginal branch. There is a long proximal dissection flap with reduced TIMI 1 flow in the distal vessel. The patient settled with conservative treatment and was discharged home seven days after admission.
Type 2
Diffuse long smooth tubular lesions (due to intramural haematoma) with no visible dissection plane that can result in complete vessel occlusion. Lesions are typically >30 mm in length with an abrupt change in vessel diameter between normal and diseased segments. There is no response to intracoronary nitrates and there are no atherosclerotic lesions in other coronary segments.
Type 3
Multiple focal tubular lesions due to intramural haematoma that mimic atherosclerosis. Intravascular imaging is required to make the diagnosis.
SCAD is often under-recognised and incorrectly classified as due to atherosclerosis if diagnosis is reliant upon visualisation of the classic dissection flap. [5] Type 2 SCAD is the most common, resulting in compression of the lumen due to intramural haematoma with no intimal flap (67% of cases). Type 1 SCAD with classic dissection flap occurs in 29%, and Type 3 SCAD mimicking atherosclerosis is relatively uncommon, occurring in 4% of cases. [6] Therefore, relying upon the identification of contrast wall staining (Type 1 SCAD) on coronary angiography would result in a significant number of cases being missed. Imaging of the vessel wall with intravascular ultrasound (IVUS) or optical coherence tomography (OCT) is required to make the diagnosis of Type 2 and Type 3 SCAD. Diffuse long coronary lesions in a young female patient with otherwise smooth coronary arteries should always promote consideration of intramural haematoma in the differential diagnosis.
The left anterior descending artery is the most frequently affected vessel, with multivessel dissections in up to 20%-25% of cases. Coronary dissections are more common in the mid- to distal segments, often involving side branches. Eleid et al. showed that coronary artery tortuosity on coronary angiography is more common in patients with SCAD (78% vs. 17% in controls), and severe coronary tortuosity is a marker for recurrent SCAD events. [7]
Patients with SCAD are thought to have more fragile coronary artery walls. Meticulous attention to angiographic technique is required to avoid catheter-induced dissection. Deep catheter intubation should be avoided, with careful monitoring of pressure traces. Gentle contrast injection to achieve adequate vessel opacification is important to minimise the risk of dissection propagation.
Management
Conservative management is preferred in stable patients with SCAD as most dissected segments will heal spontaneously (Figure 2). Medical therapy is based upon opinion, with no randomised clinical trials in this area. Initial treatment is similar to standard ACS patients with the use of dual antiplatelet agents, heparin and beta-blockers to preserve patency of the true lumen and prevent thrombotic occlusion. Glycoprotein IIb/IIIa inhibitors have also been used without complications. However, these agents could potentially delay healing of the intramural haematoma and lead to dissection extension. Thrombolytic agents should not be used due to an increased risk of bleeding and extension of intramural haematoma.
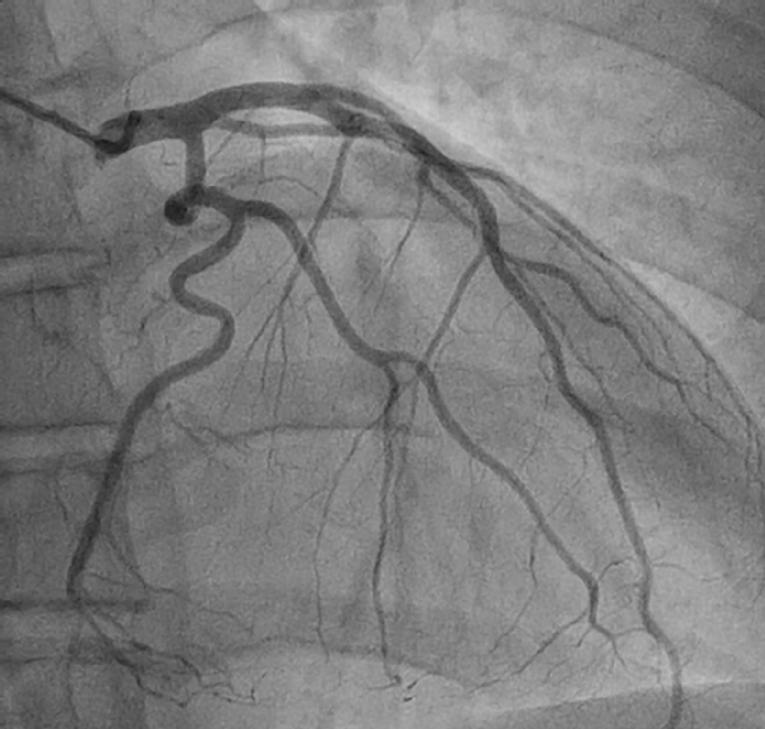
Figure 2. Repeat coronary angiogram for the same patient as in Figure 1 at three months post-discharge, showing complete vessel healing with no residual dissection.
Dual antiplatelet therapy with aspirin and clopidogrel is generally accepted, with no data on the role of more potent antiplatelet agents, such as ticagrelor and prasugrel. Although statins are important for ACS treatment in patients with atheroma, the benefit in SCAD is unknown.
Beta-blockers are recommended in all patients, with the potential to reduce arterial shear stress, facilitate healing and reduce long-term recurrence.
Indications for revascularisation include: complete vessel occlusion with thrombolysis In myocardial infarction (TIMI) 0 flow which is unlikely to resolve with medical treatment alone, left main stem involvement, ongoing ischaemia, recurrent chest pain, haemodynamic instability and sustained ventricular arrhythmias.
PCI is the preferred revascularisation strategy, but is associated with significant challenges and has reported success rates of <50%. [4,6] Technical difficulties include negotiating the guidewire into the true lumen, dissection or haematoma extension and side branch occlusion. Stent placement can result in haematoma propagation and loss of vessel flow. Coronary angiography provides poor visualisation of intramural haematoma, and intravascular imaging with OCT or IVUS is recommended in all PCI cases. OCT has superior resolution to IVUS: it can confirm guidewire position in the true lumen, detail the site of intramural haematoma and intimal tear, facilitate appropriate vessel sizing and optimise stent expansion. [8]
A conservative approach to stent implantation is preferred, with stenting of the proximal segment to seal the dissection entry, if present, and to limit the risk of dissection/haematoma propagation. Distal dissections can be left untreated if coronary flow is good with no significant obstruction. Late follow-up has demonstrated that these spontaneous distal dissections heal with reabsorption of the intramural haematoma. Drug-eluting stents are typically used in cases involving long lengths of stents to reduce the risk of restenosis. However, there is an increased risk of stent malapposition following reabsorption of the intramural haematoma, which may predispose to late stent thrombosis.
Bioresorbable vascular scaffolds (BVS) offer a promising solution by providing temporary lumen support whilst allowing vessel healing with long-term restoration of normal vessel architecture. Theoretically, BVS may reduce the risk of late stent malapposition by avoiding the use of a permanent metallic stent, although this has not been formally tested in clinical studies.
CABG is considered for patients with left main stem dissections or when PCI has been unsuccessful or is not technically feasible. The rate of emergency CABG for PCI failure is significant, varying between 10% and 13% in reported series. [4,6] This would suggest that PCI for SCAD should be performed, if possible, at centres with on-site cardiac surgery.
In-hospital results following CABG for SCAD are excellent, with a reported mortality of <2%. Follow-up angiographic studies have shown high rates of graft occlusion, possibly due to competitive flow in the native vessel or technical difficulties with distal graft anastomosis. In a single study with follow-up angiography at a median of 3.5 years, only five out of 16 grafts were shown to be patent. [6]
Prognosis
Two single-centre registries have provided important data on the natural history and prognosis of SCAD. Shaw et al. reported the results of a single-centre Canadian registry of 164 patients (mean age 52, 92% female) with SCAD. [4] Some 80% of patients were treated conservatively on initial presentation, and the in-hospital recurrent MI rate in this group was 4.5%. In patients treated conservatively who underwent delayed elective coronary angiography (≥26 days after the index event), all had spontaneous angiographic healing. Interestingly, early angiography (within 20 days) showed persistence of the coronary dissection, suggesting that the healing process is relatively slow. In the 33 patients who underwent PCI, complete success was only achieved in 36.4%. Over half of the patients undergoing PCI had procedural extension of the dissection including, in some cases, involvement of the left main stem. Only six out of the 164 patients underwent CABG, including three for failed PCI, with guide catheter-induced left main dissection in two cases. There were no in-hospital mortalities. The two-year MACE rate was 10%-17%, mainly driven by recurrent SCAD events.
Tweet et al. reported the results for 189 patients with first presentation of a SCAD episode. [6]. Women made up 92% of them, with a mean age of 44 years. Ninety-four patients were treated conservatively, with 10% experiencing SCAD progression at a mean of four days after the initial admission requiring intervention. These findings are in keeping with previous reports suggesting careful in-hospital monitoring for up to one week due to the risk of recurrent events. This is in contrast to atherosclerotic ACS cases where guidelines emphasise an early intervention and discharge approach. The PCI failure rate was 53%, underscoring the suboptimal results of PCI in this challenging group of patients.
Long-term survival was excellent, with only one death during a median follow-up of 2.3 years, although the risk of recurrent SCAD events was significant (27% at five years). Interestingly, 75%-90% of recurrent SCAD events involved coronary segments not affected at the time of the initial presentation. This would suggest that the risk for SCAD involves the entire epicardial coronary artery circulation and is not limited to isolated segments.
Collectively, these results would suggest excellent in-hospital and long-term survival, with a significant risk of future SCAD events. Therefore, patients should be warned about the risks of SCAD recurrence. At present, there is no effective treatment to reduce long-term risk.
Fibromuscular dysplasia (FMD)
FMD is a rare condition that affects medium-sized arteries, with abnormal thickening of the vessel wall causing stenosis and aneurysm formation. [9] It can involve all arterial beds, and is most commonly seen in the renal and carotid arteries. Most patients are female, aged between 20 and 60 years. The cause of FMD is unknown, and diagnosis is made by angiography (invasive or non-invasive).
The classic finding is of multifocal disease with a 'string of beads' appearance due to fibromuscular ridges causing arterial stenosis alternating with arterial dilatation. Focal disease is less common and results in localised tubular narrowing.
The first reported association between SCAD and FMD was made in 2005 by a group in Vancouver, Canada. [10] They described a case series of seven women, all presenting with acute MI, renal FMD and a distinctive pattern of coronary artery disease on angiography. This consisted of single-vessel (LAD in six and RCA in one) mid- to distal diffuse obliterative disease, with abrupt demarcation from proximal normal segments. Intravascular imaging was not performed.
The same group published results for 50 patients with similar presentation and angiographic findings. [11] All patients underwent systematic screening for FMD with the performance of invasive or non-invasive angiography of the renal, iliac and cerebral vessels. FMD was detected in 86% of patients with 42% having FMD in two or more vascular territories. The high pickup rate of FMD in this study may have been due to the frequent use of invasive angiography, with 76% of patients undergoing invasive renal angiography. CT or MR angiography is recognised to be less sensitive than invasive angiography in detecting mild FMD abnormalities.
The high prevalence of FMD in patients with SCAD suggests a direct causative association. However, additional studies are necessary for confirmation, and it is difficult to see how this will alter current management unless there is specific treatment for FMD or difference in prognosis.
Pregnancy-related SCAD
SCAD during pregnancy is uncommon, but can lead to acute myocardial infarction. Cases tend to occur within six weeks of delivery, but have been reported during early pregnancy and as late as 18 months postpartum, especially in patients still breastfeeding. [12] Pregnancy-related SCAD accounts for a small proportion (less than 5%) of all SCAD cases and is a less important cause of SCAD than originally reported. Hormonal changes during pregnancy with high progesterone levels can result in weakening of the vessel wall. Increased cardiac output and circulatory volume, along with the acute haemodynamic stress of childbirth can lead to pregnancy-related SCAD. Patients should be screened for underlying connective tissue disease or chronic inflammatory conditions. Most patients have a history of multiple pregnancies, and clinical outcomes are thought to be worse with larger infarcts, lower ejection fraction, more left main stem involvement and cardiogenic shock at presentation, compared with non-pregnancy-related SCAD. [13] The risk of recurrence is thought to be significant, with one small study showing that pregnancy-related SCAD occurred in one out of seven patients who became pregnant from a 266-patient SCAD registry. This patient developed ST-elevation MI nine weeks postpartum due to left main stem dissection requiring emergency CABG. [12] Therefore, women of a reproductive age with a history of SCAD due to any cause should be carefully counselled regarding the risk of recurrent events, especially if they are planning a future pregnancy.
Conclusions
SCAD is an important cause of ACS, particularly in young women without traditional cardiovascular risk factors.
The pathophysiology and treatment are different to ACS caused by plaque rupture or erosion.
Contrast staining of the vessel wall (Type 1 SCAD) is the most recognised finding on coronary angiography. However, Type 2 SCAD due to intramural haematoma is the commonest presentation and can be easily missed. Typical angiographic findings in Type 2 SCAD include long mid- to distal subtotal coronary occlusions without contrast staining of the vessel wall. There is often an abrupt change in vessel calibre between normal and diseased segments. Intravascular imaging is required to confirm the diagnosis.
Most coronary dissections will heal spontaneously, and conservative treatment is recommended for uncomplicated cases.
Patients with left main stem involvement, complete vessel occlusion, ongoing chest pain or haemodynamic instability will require coronary revascularisation. PCI results are suboptimal in this challenging group of patients.
PCI should be performed by experienced operators, with the use of intravascular imaging and preferably with on-site surgical backup due to the increased risk of complications.
Many patients with SCAD will also have FMD, and patients should have imaging of additional arterial beds to screen for FMD. It is not known whether this is a causative association.
Although long-term prognosis is excellent, the risk of recurrent SCAD events is significant, with an average rate of 5% per year.


 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.