Abbreviations
CIEDs cardiovascular implantable electronic devices
CRT cardiac resynchronisation therapy
ICD implantable cardioverter-defibrillator
LCP leadless cardiac pacemakers
LV left ventricle
PM pacemaker
RAO right anterior oblique
s-ICD subcutaneous implantable cardioverter-defibrillator
TPS transcatheter pacing system
Introduction
Pacemakers were developed in the 1950s, primarily to treat life-threatening heart block. They were large external devices connected to epicardial electrodes placed surgically. Although life-sustaining, these devices were plagued by many problems including short battery life, lead failures, and infections, due in part to externalisation of part of the system [1]. With advances in technology, size was reduced significantly to allow the first implantable pacemaker; however, it is now more than 50 years since transvenous leads were developed and first used with implantable devices [2]. These early pacemakers had very limited functionality and only performed asynchronous pacing. Rapid advances have been made over the past five decades, including dual-chamber pacing, rate response algorithms, remote control devices, improved battery technology and cardiac resynchronisation therapy.
Pacemaker leads, which connect the chest wall of a pacemaker to the pacing electrode in the heart, are the weakest part of pacing systems, often necessitating their risky removal and replacement. In addition, transvenous leads provide a portal into the vascular space, which increases the risk of infection. Patients with traditional pacemakers are also susceptible to haematomas and pocket infections in the chest wall where the generators lie. Thus, a self-contained leadless pacemaker which can be placed directly into the heart is an appealing prospect.
Current leadless cardiac pacemakers
Reports of two recent non-randomised, industry-sponsored studies of leadless cardiac pacemakers (LCP) have now been published and have evaluated the Nanostim device (St. Jude Medical, St. Paul, MN, USA) [3], and the Micra device (Medtronic, Minneapolis, MN, USA) [4] (Figure 1).
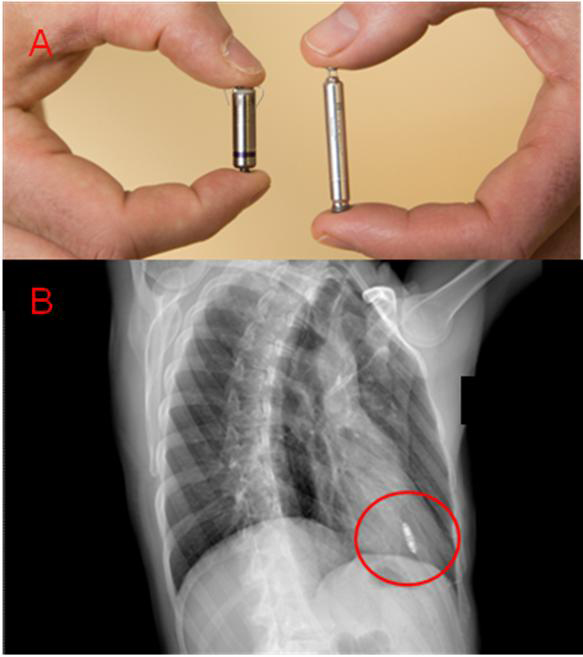
Figure 1. A) The volume of each LCP is approximately 1 cm3. Each attaches to the right ventricle — the Micra (on the left) with tines, the Nanostim (on the right) with a helical wire screw. B) Example of chest X-ray after LCP implant (Micra) in the right anterior oblique (RAO) projection.
Both devices are implanted in the same setting as traditional pacemakers (i.e., in a cardiac catheterisation laboratory or operating room), using sedative medications and local anesthesia. However, the entire procedure for both mechanisms is faster (20 to 45 minutes, depending on the experience of the specialist) compared to the 60-minute procedure for the insertion of traditional pacemakers. After placing a sheath in the femoral vein in the groin, the pacemaker is delivered to the apex of the right ventricle using a steerable catheter. The procedure is guided by fluoroscopy (Figure 2). If the position is suboptimal, both leadless pacemakers are repositionable and retrievable in the short term using a specific catheter. Moreover, a steroid-eluting tip is incorporated to reduce inflammation. After pacemaker implantation, a closing bandage is used for the access site in the groin.
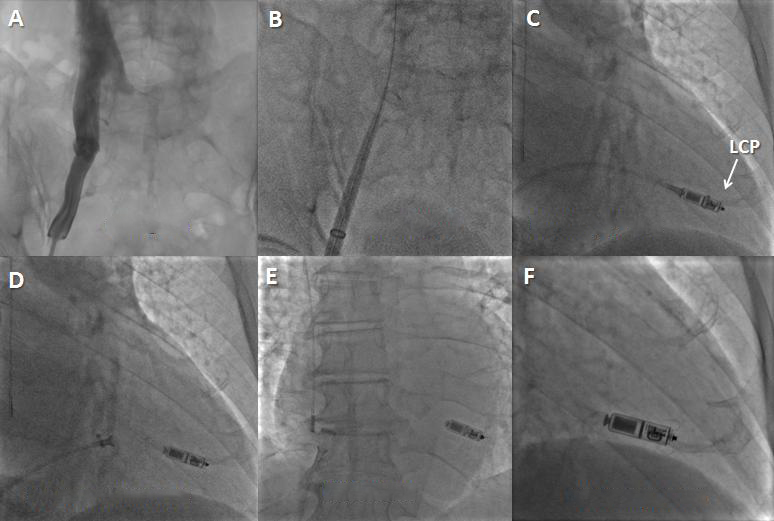
Figure 2. Leadless cardiac pacemaker (LCP) implantation steps. A) A venogram may be performed (optional). B) The introducer is placed up to the heart through the venous system. C) The LCP is positioned into the RV by deflecting the delivery system and placed in the right ventricle. The protective cover is pulled back to expose the LCP. D) The pacemaker is undocked from the delivery catheter while a tethered connection is maintained. In case the position is suboptimal, the LCP can be re-engaged and repositioned. E) & F) The LCP is released (E) in the final position. In this LCP (Micra device; Medtronic) its active fixation mechanism is made by tines.
Both systems offer pacing features similar to conventional VVIR pacemakers including rate response algorithms, thus atrioventricular synchrony is not allowed. Although these devices are significantly smaller than conventional pacing systems, the predicted longevity is 10 years. This is comparable to the longevity of a standard pacemaker and is made possible by a design optimising both energy consumption and the electrode/tissue interface. Both of these studies met their primary safety and efficacy goals. The short-term performance of the leadless pacemakers (at six months), including the persistence of good thresholds and sensing, proved to be excellent in both studies.
Unanswered questions
There are a number of questions that remain to be answered regarding this new technology. Will the device continue to perform at a high level in the long term and match the reliability of current pacemaker pulse generators? Will there be any long-term thrombogenic complications associated with an indwelling right ventricular pacemaker? Moreover, the rates of dislodgement of leadless devices over time are unknown and are of potential concern.
In the event of battery depletion, removal of the leadless pacemaker may be unnecessary, as an option may be simply to implant an additional device. Abandoning the device and implanting a second unit is probably a viable option for many patients at elective replacement, given the small size of the unit, 10-year battery life, and mean age of 70 years for initial implantation. However, how this will affect cardiac function, and how many additional devices may be implanted, remains to be determined.
Because of its design, it is expected that retrieval of the LCP will be a rare event. However, it is anticipated that there may be some circumstances in which the LCP may need to be retrieved, for example high thresholds shortly after implant or infection, although these new devices will probably be associated with a lower overall risk of infection. To facilitate retrieval, the LCP is designed with a feature on the proximal end of the device to allow a snare to capture the device. Animal studies and case reports have shown that it is possible to retrieve the device up to 28 months after implantation, using a custom sheath combined with market-released tools [5,6]. However, it is not known if it is possible to retrieve the device safely after it has been in place for more years. Device follow-up will clarify the long-term LCP performance and the related necessity of transvenous removal.
Future developments
To date, these newer devices can be used only for single-chamber ventricular pacing. However, the next generation of leadless devices is briefly taken in account next. Further studies are required to reach a definitive conclusion on the safety and the performance of these new systems.
Left ventricular resynchronisation leadless systems
There is a growing interest in left ventricular endocardial pacing, which has been discovered to be better than traditional epicardial stimulation in the setting of cardiac resynchronisation therapy (CRT) [7]. In some studies, permanent left ventricular endocardial pacing has been achieved by using a conventional lead placed in the left ventricle (LV) via a transseptal puncture [8,9].
More recently, a novel leadless pacing technology has been developed, which may address some of the issues associated with the current CRT delivery as well as potentially overcoming the safety limitations in the chronic use of conventional leads implanted in the LV [10]. The Wireless Endocardial Stimulation (WiSE) Technology (EBR Systems Inc., Sunnyvale, CA, USA), which is not available at the time of publication of this article in the United States, consists of a small electrode implanted in the LV that paces the heart via electrical energy. The electrode generates electricity by converting an ultrasound signal received from a small transmitter placed between the ribs. Another advantage of leadless pacemakers is their small size, which makes them easier to implant than a transcatheter pacing system (TPS).
Thus, chronic left ventricular endocardial pacing may address the limitations in the selection of an LV pacing site and provide improvements in CRT effectiveness. In fact, two independent randomised controlled trials, TARGET (Targeted Left Ventricular Lead Placement to Guide Cardiac Resynchronisation Therapy) and STARTER (Speckle Tracking Assisted Resynchronisation Therapy for Electrode Region), have demonstrated that better targeting of the left ventricular pacing site (at the site of latest contraction or ventricular activation) leads to improvements in clinical response, including freedom from heart failure, hospitalisation, and mortality [11,12].
The feasibility of providing an endocardial stimulation for CRT with leadless technology was successfully demonstrated in the WiSE CRT study [13]. This study demonstrated a significant subjective improvement in the patients’ functional status and a significant increase of LV ejection fraction. However, the study was terminated prematurely for safety reasons, and so the delivery system was redesigned. Then the SELECT-LV study suggest that the delivery system modifications permit atraumatic implantation of the receiver electrode onto the left ventricular endocardial surface (Presented at the Heart Rhythm Society Annual Scientific Sessions, May 2016 in San Francisco, CA, USA. Reddy VY et al. Leadless LV Endocardial Stimulation for CRT: Final Outcomes of the Safety and Performance of Electrodes Implanted in the Left Ventricle [SELECT-LV] Study. Abstract AB17-02).
There remain several technology-specific concerns that require consideration. First, although the left ventricular receiver component of this system is indeed leadless, the system does require the concomitant conventional implantable right ventricular pacing devices.
Second, it is theoretically possible that long-term ultrasound energy exposure to subcutaneous or myocardial tissue in humans may have unintended adverse consequences. Third, there may be untoward effects of external (environmental) interference and changes in the acoustic window on the system’s sensing or pacing performance.
Combination of leadless pacemaker and subcutaneous defibrillator
In recent years, major advances have been achieved in entirely subcutaneous ICD (s-ICD) systems [14] that obviate intracardiac leads and hence carry the potential to reduce associated complications. The s-ICD is establishing its role as a viable alternative, particularly as primary prevention in non-pacing-dependent younger patients. Unlike standard implantable cardioverter-defibrillators (ICD), the s-ICD is devoid of anti-bradycardia pacing capabilities. Nevertheless, certain patients may benefit from leadless devices with both pacing and ICD functions.
Recently, the case of the first patient in whom the leadless pacemaker and s-ICD were used in combination has been described [15]. This patient had been implanted with an s-ICD system and after some time presented a complete atrioventricular block, but he had no vascular access for transvenous lead insertion, so a leadless pacemaker was inserted. When these two technologies are used together, pacemakers must be programmed to stimulate in a bipolar mode in order to avoid double counting by the s-ICD. Although VF was not re-induced, an 80 J shock was delivered by the s-ICD on QRS timing, which did not result in mode reversion, shutdown, or dislodgment of the pacemaker. Moreover, no noise was perceived by the s-ICD during pacemaker interrogation and programming, even when programmed to maximum output stimulation. This strategy carries the potential of pacing and defibrillation for patients in whom standard transvenous approaches are not feasible or desirable.
The leadless devices may ultimately obviate conventional pacing technologies and enhance arrhythmia sensing by the s-ICD which will be able to incorporate intracardiac electrogram data to confirm ventricular arrhythmias, reducing the oversensing of T waves, myopotentials, or non-biologic noise associated with such ICDs.
Multi-chamber leadless pacing systems
Further development of both leadless pacemakers for multi-chamber pacing may occur pending positive results from ongoing studies. Multi-device systems can introduce unique challenges for implementing such multi-chamber therapy. In multi-device systems, two separate devices may be responsible for sensing cardiac events in different chambers and delivering electrical stimulation to the different chambers. In some instances, each of the devices may be able to detect and/or deliver electrical stimulation to one chamber of the heart. The multiple devices of such systems may be configured to communicate sensed cardiac events and other information to the other devices in order to deliver electrical stimulation to the various chambers safely and effectively.
Economic considerations
It is anticipated that leadless pacemakers will be more expensive than a transcatheter pacing system (TPS). In Europe, the estimated cost for the Nanostim pacemaker and implantation procedure is €11,500. The cost of the retrieval of the pacemaker (if necessary) shortly after implantation is €6,000 [16]. However, savings generated from the shorter procedure time and fewer healthcare resources used to manage lead and chest pocket complications could help offset the additional cost.
Another economic consideration is the battery life comparison. Based on the International Organisation for Standardisation (ISO) standard (2.5 V, 0.4 ms, 600 U, 60 beats/min, and 100% pacing), the battery longevity of a TPS is approximately one half that of the LCP (4.7 vs. 9.8 years). Even considering the use of capture management to minimise battery drain, the projected battery life for the LCP at nominal settings (1.5 V, 0.24 ms, 60 beats/min with an impedance load of 500 ohms and 100% pacing) would be longer than the TPS one (14.7 vs. 9.6 years) [17].
A longer battery life means less need for replacement for LCP compared with TPS. This would save healthcare resources from the additional cost for a new device and patients from the infection risk related to the procedure. A complete study comparing the costs of LCP and TPS is still missing, but we can postulate an economic return of LCP both for hospital resources and patient health.
Discussion
Predicting the role of this device in pacemaker therapy is a challenge, as it is for most new devices. In the United States of America and most European countries, ~20% to 30% of patients receive ventricular pacemakers primarily for chronic atrial fibrillation, and this was largely the population implanted in the current trial. However, in less developed countries, this proportion is often ≥50% [18]. In addition, pacemaker use is limited in many areas of the world, in part because of the lack of implantation expertise. The steep learning curve for the leadless pacemaker implantation and the more common skill set of femoral vascular access and sheath manipulation suggest that this approach could fill an unmet need. Consequently, the adoption of this therapy in such countries could be high, particularly if the cost is competitive with traditional pacing systems.
Future developments of leadless pacing make it easy to envisage its important role in future cardiovascular implantable electronic devices (CIEDs). The ability to communicate with a second device placed in the right atrium would result in dual-chamber pacing capabilities. Similarly, communication with a subcutaneous ICD would allow both bradycardia and anti-tachycardia pacing, and would probably improve rhythm discrimination. The potential safety of placing leads in the left ventricle in the absence of anticoagulation is unclear, but endocardial stimulation with a leadless technology could be a novel developing tool for cardiac resynchronisation therapy.
Conclusion
All of these possibilities point towards a bright future for leadless pacing with the likely possibility that the devices of the future will be largely devoid of intravascular leads. As such, leadless cardiac pacemakers could become the future of pacing in many types of device.



 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.