Introduction
Diabetic cardiovascular autonomic neuropathy (DCAN) was first described in 1975 by Low et al, after determining that diabetic neuropathy also compromised autonomic control of the cardiovascular system, as evidenced by orthostatic hypotension (OH) and abnormal autonomic responses to different stimuli [1]. This condition is associated with an increased morbidity and mortality, conferring a poor prognosis [2]. Hyperglycemia and hyperinsulinemia induce metabolic alterations that lead to oxidative stress and demyelination [3]. Good glycemic control, control of cardiovascular disease (CVD) risk factors and pharmacologic modulation of oxidative stress and autonomic dysregulation reduce its progression and the increased risk of death from this condition [3]. Actually, there are no formal recommendations from endocrinology and diabetes scientific societies, and only one guideline from an expert panel on how to address this issue [4]. This article reviews the key topics of DCAN and its impact in the natural history of disease.
Epidemiology
Diabetes mellitus (DM) is a chronic non-communicable disease of high public health impact in all countries whatever their income level with a high global burden that has escalated into an epidemic. The International Diabetes Federation (IDF) has estimated a global prevalence of 8.8% (415 million people), with an expected increase to 10.4% (642 million people) in 2040 [5]. This high prevalence is a consequence of the ageing of the population and industrialization with the consequent acquisition of a sedentary lifestyle and unhealthy habits, leading to obesity, which is the most important risk factor for this condition [5, 6]. T2DM and its complications are the main causes of premature deaths in many countries [5]. Its complications can compromise the macro (stroke, coronary artery disease [CAD], peripheral artery disease, cerebrovascular disease) and micro (retinopathy, nephropathy and neuropathy) vasculature, actually the main cause of non-traumatic amputations, legal blindness and advanced chronic kidney disease (CKD) requiring dialysis [5]. DCAN is one of the least understood of all complications of DM and has been defined as the presence of abnormal myocardial performance secondary to dysregulation of autonomic cardiovascular control in the absence of other causes [3, 7]. Its prevalence reaches 17% of patients with T1DM and 22% of those with T2DM, increasing to 40% in patients with insulin dependence [2, 3, 7]. There is a strong association between DCAN and mortality, with some authors reporting a steady increase over an 8-year period in comparison with a control group matched by age, sex and duration of diabetes [2, 3, 7]. In patients with advanced autonomic denervation and orthostatic hypotension the lethality rates increase to 16-35% at 5 years [2, 3, 7].
Pathophysiology
In DCAN there are disturbances in the balance of the autonomic nervous system (ANS) that result in loss of heart rate variability (HRV) and abnormalities in microvascular dynamics [3, 7, 8]. Hyperglycemia leads to sympathetic denervation, changes in myocardial autonomic neurotransmitter levels, altered expression of neuropeptides and their signaling pathways, together with an altered beta-receptor density. Hyperglycemia causes an increase in intracellular sorbitol, leading to accumulation of intracellular osmotic particles, reduction of levels of NADPH and formation of advanced glycation end-products that leads to oxidative stress, activation of endoproteases (PKC and MAPK), altered endoneural and perineural blood flow, resulting in nerve hypoxia, ischemia and axonal degeneration, together with demyelination [1, 3]. Insulin resistance impairs myocardial contractility via reduced Ca2+ influx through L-type Ca2+ channels, reverse mode Na2+/Ca2+ exchange and impairment of the PI3-kinase/Akt pathway [3, 7, 8]. These molecular changes lead to diastolic dysfunction, left ventricular hypertrophy (LVH), cardiac remodeling secondary to interstitial myocardial fibrosis, and ultimately to left ventricle (LV) systolic dysfunction [3, 7, 8]. The relative predominance of sympathetic activity at the onset of DCAN would stimulate the renin angiotensin aldosterone system (RAAS), which not only increases the hemodynamic stresses and energetic requirements of the LV by sodium retention and peripheral vasoconstriction, but may also exert direct noxious effects on cardiomyocytes that cause more impairment on the performance of the LV [3, 7, 8]. Autonomic dysfunction has also been associated with an impaired response to hypoglycemia with potentially lethal consequences. Recent studies also link hypoglycemia to progression and development of autonomic dysfunction, although the pathophysiological mechanisms of this association are not yet understood [3, 7-9].
Clinical manifestations
DCAN may be subclinical but, when symptoms appear, they are usually characterized by exacerbations and remissions [3]. In some patients, symptoms remain stable over time and fewer experience progressive deterioration [3]. It should be noted that patients with clinical DCAN usually also suffer other facets of diabetic polyneuropathy [3]. Clinical manifestations of DCAN include: resting heart rate (HR) disorders, exercise intolerance, intraoperative cardiovascular lability, orthostatic alterations in heart rate and blood pressure (BP), QTc prolongation, abnormal diurnal and nocturnal blood pressure variations, silent myocardial ischemia (SMI) and diabetic cardiomyopathy [3].
Resting tachycardia
Resting tachycardia is a common manifestation of DCAN that occurs at a relatively early stage of the disease. An HR of 90-130 beats per minute (bpm) can be observed and is associated with a reduction in parasympathetic tone followed by increased sympathetic activity as DCAN progresses. In the early stages of autonomic dysfunction, a decrease in HRV is observed. Later, when the parasympathetic limb of the autonomic nervous system (ANS) is affected, resting tachycardia appears (90-100 bpm with bursts of 130 bpm) [3, 8]. After about 5 years of latency, when sympathetic cardiac denervation develops, impairment in HR adjustment to exercise, stress and sleep emerges, and the rate returns toward normal but still remains elevated as compared with healthy individuals [3, 8]. In more advanced stages, HR becomes fixed and unresponsive as a result of complete heart denervation, indicating almost complete cardiac denervation and severe DCAN [3, 8].
However, resting tachycardia is a non-specific sign for DCAN, as may be present in several other conditions, but it can be used as a diagnostic and prognostic tool after excluding other causes [3, 8].
Exercise intolerance
Autonomic dysfunction impairs exercise tolerance, reduces response in HR and BP, and blunts increases in cardiac output (CO) in response to exercise through limited adjustability of HR, BP and CO to conditions of increased work demand [3, 7, 8). Concomitant CAD, diastolic and systolic left ventricular dysfunction may also contribute to exercise intolerance [3, 7, 8].
Orthostatic hypotension (OH)
In patients with DCAN, the transition from supine to standing position can cause abnormal responses, such as hypotension, tachycardia or even bradycardia [3, 7, 8]. Blood volume depletion due to diuretic therapy, sweating, diarrhea or polyuria and concomitant antihypertensive medication such as β-blockers, insulin, tricyclic antidepressants and phenothiazines, can also contribute to OH development [3, 7, 8]. It usually presents as dizziness, fatigue, visual disturbances, syncope, but may also remain asymptomatic [3, 7, 8]. Symptoms are present in 4-32% of diabetic patients, depending on diagnostic cut-offs for blood pressure fall (20 or 30 mmHg) and population characteristics [3, 7, 8]. Postural orthostatic tachycardia syndrome is diagnosed when a greater than 30 bpm increase or a heart rate of over 120 bpm is documented within 12 min of transition from the supine to the upright position during tilt test; however, the exact pathophysiological mechanisms implicated in these responses have not yet been fully elucidated [3, 7, 8], as it is an independent prognostic factor for CVD and all-cause mortality [3, 7, 8].
QTc prolongation
The pathogenesis of QTc prolongation is multifactorial and includes imbalance in cardiac sympathetic innervation, intrinsic metabolic and electrolytic myocardial changes, LVH, CAD, and genetic factors [3, 7, 8). Reversible QTc prolongation may be induced by hyperinsulinemia, hyperglycemia and acute hypoglycemia [3, 7, 8], supporting an arrhythmic basis for the “dead in bed” syndrome and possibly a provocative role in cardiovascular events of hypoglycemia-induced sympathetic activation [3, 7, 8].
Perioperative and intraoperative complications
Patients with DCAN exhibit a two to threefold increase in perioperative morbidity and mortality, are more likely to require vasopressor support in the operation room, and are also prone to experience a BP and HR reduction during the induction of anesthesia, as well as severe intraoperative hypothermia [3, 7, 8]. The above findings can be explained by an impairment or absence of the normal vasoconstrictive response [3, 7, 8].
Silent myocardial ischemia (SMI)
SMI is a clinical entity not well clarified, believed to result from damage of the ANS pathways of pain [3, 7, 8). Upshift in anginal perceptual threshold, atypical presentation of acute coronary events and/or asymptomatic course of myocardial infarction (MI) can be facets of SMI [3, 7, 8]. PET studies have detected a failure in signal transmission from the thalamus to the frontal cortex in individuals with SMI, implying that unsensed ischemia might not only be a matter of impaired peripheral neural conduction but also be a result of central nervous system disorders [3, 7, 8]. DCAN is independently associated with increased risk for developing SMI (OR=6.5, 95% CI: 1.3-7.9), especially when combined with other cardiovascular risk factors such as microalbuminuria and slow HR recovery after exercise [10]. Patients with DCAN show delayed onset of angina symptoms after the appearance of ECG ischemic changes during exercise testing or very often develop atypical symptoms such as unexplained fatigue, confusion, hemoptysis, nausea, vomiting, sweating, arrhythmia, coughing and dyspnea [3, 7, 8]. Therefore, presence of atypical symptoms should be regarded as of myocardial origin unless proven otherwise [3, 7, 8].
Abnormal blood pressure regulation
Non-diabetic subjects present with predominance of vagal tone and decreased sympathetic tone at night, associated with reduction in nocturnal BP. In diabetic DCAN, this pattern is altered, resulting in nocturnal sympathetic predominance during sleep and subsequent nocturnal hypertension, also known as non-dipping and reverse dipping [3, 7, 8]. These are associated with a higher frequency of LVH and fatal and severe non-fatal cardiovascular events in DCAN subjects with a two to eightfold increase in risk of cardiovascular or renal events in some longitudinal studies [3, 7, 8].
Diagnosis
Early detection of DCAN is of paramount importance, since it can lead to prompt therapeutic interventions, resulting in a significant survival benefit [3, 4, 7, 8]. Medical history and physical examination are inadequate for the diagnosis which requires specific diagnostic tests. Subclinical DCAN may be detected within 1 year of diagnosis in T2DM and within 2 years of diagnosis in T1DM [8].
In early 1985, Ewing et al described five simple tests for non-invasive autonomic evaluation [11]: heart rate response to breathing, heart rate response to standing, Valsalva maneuver, blood pressure response to standing and blood pressure response to sustained handgrip.
Conventional cardiovascular autonomic reflex tests (CARTs) are non-invasive, safe, clinically relevant (they correlate with tests of peripheral nervous system function), easy to perform, sensitive, specific, reproducible, and standardized. Therefore, they are considered the gold standard measures of autonomic function [3, 4, 7, 8].
While CARTs (Table 1) have been widely used since their introduction, there is no evidence on the superiority of one test over another when it comes to assessing DCAN [3, 4, 7, 8]. However, the HR response to deep breathing is the most commonly utilized, because of its high reproducibility and specificity ∼80% and its ease of use [3, 4, 7, 8]. The orthostatic hypotension test has low sensitivity and high specificity, making it suboptimal for diagnosis [3, 4, 7, 8]. Other methods such as cardiac sympathetic imaging, microneurography, occlusion plethysmography, and baroreflex sensitivity are currently used predominantly in research settings but may find a place in the clinical assessment of DCAN in the future [3, 4, 7, 8]. However, cardiovascular tests based on HR response to deep breathing, lying to standing and Valsalva maneuver, and BP response to standing (OH test) are an essential and irreplaceable part of DCAN diagnosis [3, 4, 7, 8].
Table 1. Classification of cardiovascular autonomic reflex tests.
|
SYSTEM |
RELEVANT TEST |
|---|---|
|
Parasympathetic |
HR response to breathing |
|
HR response to standing |
|
|
Spectral analysis of HRV high-frequency domain |
|
|
Total spectral power of HRV |
|
|
Sympathetic |
SBP response to standing |
|
DBP response to handgrip |
|
|
QTc prolongation |
|
|
Spectral analysis of HRV low-frequency domain |
|
|
[123] MIBG, [11C]-HED cardiac imaging |
|
|
Microneurography |
|
|
Catecholamine levels |
|
|
Both parasympathetic and sympathetic |
Resting HR |
|
Valsalva ratio |
|
|
Nocturnal BP dipping |
|
|
Baroreflex sensitivity |
Modified and reproduced with permission from Karayannis G, Giamouzis G, Cokkinos DV, Skoularigis J, Triposkiadis F. Diabetic cardiovascular autonomic neuropathy: clinical implications. Expert Rev Cardiovasc Ther. 2012;10[6):747–65. Abbreviations: HR: heart rate; HRV: heart rate variability; SBP: systolic blood pressure; DBP: diastolic blood pressure; QTc: corrected QT interval; MIBG: metaiodobenzylguanidine; HED: metahydroxyephedrine.
Among the physiological factors affecting the test results, the most important are: age, respiratory pattern, body position and duration of supine rest, resting heart rate and BP, physical exercise within 24 hrs, coffee, alcohol and cigarette consumption, meals, and drugs [3, 4, 7, 8].
In the case of altered cardiovascular tests in the baseline evaluation, it is advisable to repeat the tests annually in order to confirm the diagnosis of DCAN and evaluate its progression [4]. Moreover, even in the absence of alterations of cardiovascular tests, it is advisable to repeat the tests annually in diabetic patients with poor glycemic control, high cardiovascular risk and microangiopathic complications, whereas in the other patients a longer interval is recommended [3, 4, 8].
Apparently, not all diabetics require autonomic function assessment [3, 4, 8]. Table 2 contains a summary of indications for testing.
Table 2. Indications for CARTs
|
Indications for CARTs |
|---|
|
At time of diagnosis in T2DM |
|
5 years after T1DM onset |
|
Poor glycemic control [HbA1c >7%) |
|
Patients beginning a moderate to high-intensity exercise routine |
|
Patients with symptoms suggesting autonomic dysfunction |
|
Patients with major CVD risk factors (HTN, DLP, smoking) |
|
Micro or macroangiopathic complications |
|
Perioperative risk assessment before major surgery |
Abbreviations: CARTs: cardiovascular autonomic reflex tests; CVD: cardiovascular disease; DLP: dyslipidemia; HbA1c: glycated hemoglobin; HTN: hypertension; T2DM: type 2 diabetes mellitus; T1DM: type 1 diabetes mellitus.
Staging of DCAN
The available information regarding the duration required to progress from an earlier to a later stage of impairment is scant and it is not documented whether a progression to OH and symptomatic forms invariably occurs in all patients [3, 4, 7, 8]. Following the 8th International Symposium on Diabetic Neuropathy in 2010, criteria for diagnosis and staging of DCAN were defined in the Subcommittee of the Toronto Consensus Panel Statement (Figure 1) [4]. Accordingly, only one abnormal CARTs result is sufficient to diagnose possible or early DCAN, two or three abnormal tests indicate definite or confirmed DCAN, and concurrent OH indicates severe/advanced DCAN [4]. Progressive stages of DCAN are associated with an increasingly worse prognosis [1, 4, 7, 8, 10].
Figure 1. DCAN stages
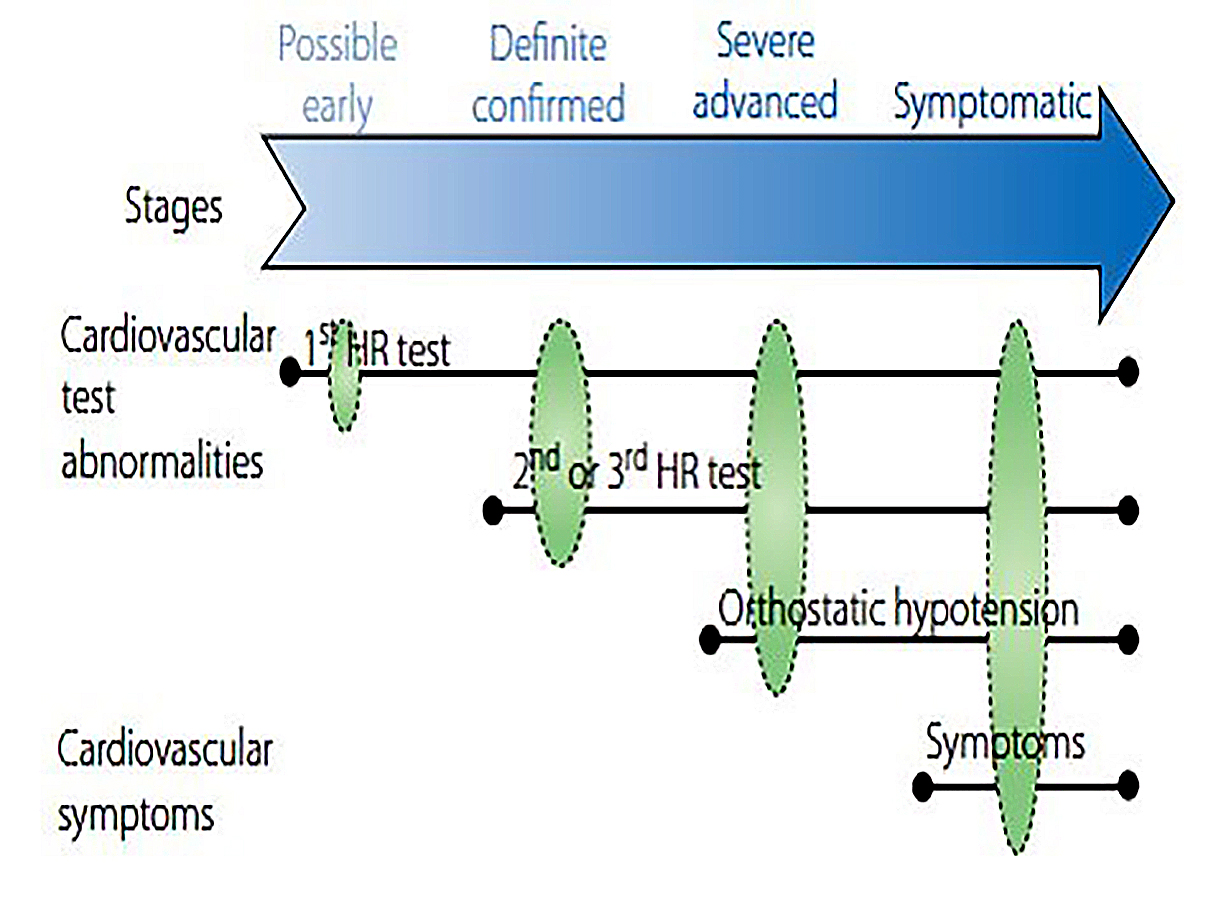
Reproduced with permission from Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, et al. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011 Oct;27[7):639-53. Abbreviations: CAN: cardiac autonomic neuropathy; HR: heart rate.
Prognosis
DCAN is a complication that confers a higher morbidity and mortality from cardiovascular and all causes [1, 4, 7-13]. The prognostic importance of DCAN presence was initially identified in the early 1980s, when DCAN was associated with a nearly fivefold increase in mortality risk [11]. Since then, a large number of publications have verified this association [4, 9, 10, 12, 13]. Among 2,787 T1DM patients, DCAN was a significant predictor of 7-year mortality, exceeding the relative effect of the traditional CVD risk factors [16].
The pathophysiological mechanisms contributing to increased mortality are not yet clear. These include: SMI, disturbances in coronary flow regulation, increased HR, diastolic and systolic LV dysfunction, QTc prolongation and increased risk for arrhythmias, diminished sense of hypoglycemia and difficult recovery due to dysregulation of compensatory endocrine mechanisms, acceleration of renal impairment, alterations of circadian BP cycle, increased susceptibility to medications that cause respiratory depression, impaired respiratory response to hypoxia, increased sympathetic activity and increased calcification of the coronary arteries (1, 4, 7-16).
A meta-analysis of 15 longitudinal studies, which included a total of 2,900 patients followed up for 16 years, showed that the diagnosis of DCAN based on at least two abnormal CARTs results determined a relative risk of mortality of 3.65 (95% CI: 2.66-4.47), even after correction for multiple confounding factors [12]. Moreover, the pooled relative risk of mortality in clinic-based studies that used more than one index was considerably higher than in studies that used only one [12, 16, 17]. More important is that prognostic information for death and/or cardiac events in terms of incremental risk, derived from DCAN diagnosis, outweighs that offered by perfusion defects or by the presence of SMI [10, 14, 15].
The association of DCAN with nephropathy [12, 14, 15], post MI [12], QTc prolongation [9, 12, 16, 17] and neuropathy [9, 12-17] increases the risk for mortality and CVD morbidity. Moreover, DCAN was associated with LV systolic and particularly diastolic dysfunction in the absence of cardiac disease [3, 4, 8].
Each manifestation of DCAN is associated with increased morbidity and mortality in these patients. Resting tachycardia [17] and OH [4] are associated with higher risk for adverse cardiac events including all-cause and CVD mortality, MI [OR=4.16, 95% CI: 1.01-17.19) and sudden cardiac death [3, 4, 9, 12-16]. When DCAN was combined with SMI, the risk was even higher (5 out of 10 had a major event) [9, 10, 12, 16], with DCAN being an independent risk factor for developing SMI [OR=6.5, 95% CI: 1.3-7.9), especially when combined with other cardiovascular risk factors such as microalbuminuria [9, 10, 12, 16].
Therapy
Poor glycemic control [3, 7, 8], high glycemic variability [3, 7, 8] and hypoglycemia [9] have been related to the development of this complication, together with the presence of multiple cardiovascular risk factors which seem to modulate the progression and types of manifestation the patients develop [3, 7-9]. A good glycemic and CVD risk factor control avoiding glycemic variability and hypoglycemia seems the most important therapeutic approach for its prevention [3, 7-9]. Once established, the avoidance of hypoglycemia with a less stringent but good glycemic control [3, 7-9] associated with a cardiovascular risk reduction strategy [3, 4, 7, 8, 18] and use of beta-blockers to counteract sympathetic hyperactivation [19] is vital to slow its progression. Once OH is present, avoidance of hypotension is essential, since a strict control of blood pressure has been related in this patient with excessive mortality [9]. Thus, in diabetic patients, BP should not be measured only in the seated position when adjusting antihypertensive treatment, and drugs with adverse autonomic consequences and potential for QTc prolongation should be avoided [3, 4, 17]. Use of RAS blockade with ARBs, ACE inhibitors and spironolactone has no proven efficacy or impact in its course [3, 7-9]. Small studies show promising results with the use of anticonvulsants [3, 4]. Several molecules with antioxidant and antifibrotic properties are being tested, looking to impact on its natural history [3, 4, 7, 8, 18, 20].
Conclusions
DCAN is a frequently forgotten and underdiagnosed microvascular complication of DM. Patients with this condition are at high risk of sustaining major cardiovascular events and death. Diagnosis is simple but requires time and skills for making and interpreting CARTs. Screening can be done in the office at low cost. Actual DM management guidelines aim to reduce CVD mortality but have forgotten to address this issue. Tailored and individualized management with avoidance of hypoglycemia and OH is important in these patients, since these events carry a high morbidity and mortality in this set of autonomic dysfunctioning patients. It is imperative to begin recognizing DCAN to accomplish a better prognosis and reduce CVD mortality among people with longstanding DM.





 Our mission: To reduce the burden of cardiovascular disease.
Our mission: To reduce the burden of cardiovascular disease.